180 Life Sciences Corp. (NASDAQ: ATNF)
180 Life Sciences Corp. (NASDAQ: ATNF) is a clinical-stage biotechnology company focused on the development of novel drugs that fulfill unmet needs in inflammatory diseases, fibrosis and pain by leveraging the combined expertise of luminaries in therapeutics from Oxford University, the Hebrew University and Stanford University.
KBLM has valued 180 Life Sciences at $175 million, with the acquisition being carried out via a share swap through which each share of 180 Life Sciences will be exchanged for one share of KBLM.
Drug Development Programs
180 Life Sciences is leading the research into solving one of the world’s biggest drivers of disease – inflammation. The company is driving groundbreaking study into clinical programs, which are seeking to develop novel drugs addressing separate areas of inflammation for which there are no effective therapies.
The company’s primary platform is a novel program to treat fibrosis and inflammation using anti-TNF, with its lead program in phase 2b/3 clinical trials with first results expected in 2021. Further clinical trials are scheduled to begin by the end of 2020. The company has two additional programs that are in the preclinical stage and are showing promising results.
- Fibrosis & Anti-TNF (Phase 2b/3 Trials): Based at the Kennedy Institute within Oxford University, the fibrosis and anti-TNF program is being led by Professor Jagdeep Nanchahal, a surgeon-scientist who has been running the phase 2 trials, and Professor Sir Marc Feldmann, a renowned immunologist and one of the pioneers of anti-TNF therapy. The program is designed to address four critical areas of inflammation:
- The phase 2b/3 trial evaluating the treatment of early stage Dupuytren’s disease (DD) is a fully grant-funded and enrolled study, with top line data expected to be available by Q4 2021.
- The phase 2b trial studying the treatment of frozen shoulder is likewise grant-funded and is scheduled to be initiated by Q3 2021.
- The phase 2 trial in post-operative cognitive deficit (POCD) is anticipated to commence in Q4 2021.
- Preclinical studies in liver fibrosis and nonalcoholic steatohepatitis (NASH) are set to begin in late 2020.
- Inflammatory Pain (Preclinical): Directed by Professor Raphael Mechoulam at the Hebrew University in Israel, this program is focused on discovering novel compounds to treat chronic inflammatory pain.
- A7nAChR (Preclinical): Led by Professor Lawrence Steinman and Dr. Jonathan Rothbard, 180 Life Sciences is seeking to develop a treatment for ulcerative colitis in ex-smokers by targeting the a7nAChR, a nicotine receptor in the body and a central factor in the body’s method of controlling inflammation.
Market Size for Anti-Inflammatory Medication
According to a study carried out by Allied Market Research, the anti-inflammatory therapeutics market is expected to grow to an approximate $106.1 billion annual market size in 2020, registering a CAGR of 5.9% during the period from 2015 to 2020.
Ranging from asthma treatments to targeting the causes of diseases such as arthritis, multiple sclerosis, psoriasis and inflammatory bowel disease, anti-inflammatory therapeutics have seen a sharp increase in usage, particularly given that they allow for medical responses that are more targeted and effective while possessing lesser side effects relative to conventional drugs.
Management Team
Professor Sir Marc Feldmann, Co-Chairman, is known to be a pioneer of anti-TNF therapy, which seeks to suppress the immune system by blocking the activity of TNF, a substance in the body that can cause inflammation and lead to immune-system diseases. As of today, anti-TNF therapy drugs have become the world’s largest drug class, with sales estimated at over $40 billion per annum. Feldmann has received seven international awards for biomedical innovation over the years, including the Crawford and Lasker awards, and he is a member of the Royal Society.
Professor Lawrence Steinman, Co-Chairman, is a scientific luminary, having discovered the role of integrins, which led to the creation of Natalizumab, a highlight effective treatment for multiple sclerosis and inflammatory bowel disease. Steinman is a member of the National Academy of Sciences and has received four international awards for biomedical innovation, including the Charcot Prize. Prior to joining 180 Life Sciences, Steinman founded Centocor, a pharmaceutical company that was sold to Johnson & Johnson for $4.9 billion.
Dr. James N. Woody, CEO, was instrumental in the discovery of Remicade as Chief Scientific Officer at Centocor. Previously, Woody founded Avidia and Proteolix, both of which were subsequently sold to Amgen, and he was a General Partner at Latterell Venture Partners. Boasting over 25 years of pharmaceutical research and management experience, Woody was also previously the general manager of Roche Biosciences, the former Syntex Pharmaceutical Company.
Aditxt Inc. (NASDAQ: ADTX)
Aditxt Inc. (NASDAQ: ADTX) is a biotech innovation company developing technologies focused on mapping and reprogramming the immune system. Aditxt’s immune mapping technologies are designed to provide a personalized immune profile. Aditxt’s immune reprogramming technologies, currently preclinical, are being developed to retrain the immune system to induce tolerance to address rejection of transplanted organs, autoimmune diseases, and allergies.
As further discussed below, the company’s first commercial product is an immune mapping technology, AditxtScore™, which is designed to provide a personalized profile of the immune system.
The company’s preclinical immune reprogramming technology, Apoptotic DNA Immunotherapy™ (“ADi™”), aims to retrain the immune system to induce tolerance, with the goal of addressing vast unmet needs in transplanted organ rejection, autoimmune diseases, and allergies. The company is developing specific ADi™ products for psoriasis, type 1 diabetes, and skin grafting.
Headquartered in Richmond, Virginia, Aditxt also operates locations in Silicon Valley and New York.
AditxtScore™
AditxtScore™ is a proprietary platform designed to provide a personalized, comprehensive profile of an individual’s immune system. The underlying technology, licensed from Stanford University through an exclusive worldwide agreement, offers a highly sensitive and accurate method of detecting and quantifying cellular responses, allowing greater specificity, quantification, and amplification of both clinical and commercial opportunities.
The company’s first commercial application of the platform, AditxtScore™ for COVID-19, delivers timely reports on vulnerability and immune status relating to SARS-CoV-2 and its known variants, giving consumers and physicians the data needed to make informed health decisions. Potential future applications will offer early detection of an array of conditions, including diabetes, cardio-metabolic maladies and hormonal imbalances.
Aditxt’s AditxtScore™ immune monitoring center in Richmond, Virginia, is operational and designed to support the anticipated increased demand for AditxtScore™ as well as related products and services. The company is currently scaling its capabilities at this location, with a goal of processing up to 10 million immune system tests/reports annually.
ADi™
ADi™ is Aditxt’s immune reprogramming platform addressing disease-causing immune responses while maintaining the immune system’s ability to combat pathogenic infection. The company is commercializing a nucleic acid-based technology called Apoptotic DNA Immunotherapy™ (ADi™) which utilizes a novel approach that mimics the way our bodies naturally induce tolerance to our own tissues (therapeutically induced immune tolerance). Aditxt believes its ADi™ technology platform can be engineered to address a wide variety of indications.
Aditxt is currently developing ADi™ products for psoriasis, type 1 diabetes and skin grafting.
Currently, immuno-tolerance is achievable through chimerism and cell-based therapy, but there is a clinical need for a more practical and cost-effective approach which:
- Can be made into a product
- Does not require additional hospitalization
- Is simple to produce and ship
Preclinical studies have demonstrated that ADi™ treatment significantly and substantially prolongs graft survival, in addition to successfully “reversing” other established immune-mediated inflammatory processes. ADi™ treatment is not expected to require hospitalization, instead being delivered as an injection in minute amounts into the skin.
IP Portfolio
Both AditxtScore™ and ADi™ are supported by a strong IP portfolio.
AditxtScore™, built upon initial technology invented, licensed from and used at Stanford University, is protected by U.S. patents encompassing methods, systems, and kits for detection and measurement of specific immune responses.
ADi™ technology is protected by seven patent families, including:
- 8 U.S. patents
- 4 pending U.S. patent applications
- 86 foreign patents and 14 pending foreign patent applications spanning the EU, Australia, Canada, Japan, China, India and Hong Kong
These patents are broadly categorized into three groups:
- Autoimmune diseases and Type 1 Diabetes
- Organ transplantation and a method of producing plasmid DNA to prevent immune activation
- Composition of matter for a tolerance delivery system for antigens of interest
Aditxt also possesses and/or in-licenses substantial know-how and trade secrets relating to the development and commercialization of its product candidates, including related manufacturing processes and technologies.
Market Overview
The potential market opportunities presented by immune monitoring and reprogramming are extensive, particularly as Aditxt continues to evaluate additional applications for the platforms.
The company’s initial focus on organ transplantation and related autoimmune response provides some insight into the potential of its approach. According to BCC Research, the global organ and tissue transplantation and alternatives market is on course to reach $120.3 billion by 2024, recording a CAGR of 7.4% from 2019. Industry data suggest that approximately 50% of all transplanted organs are rejected within 10-12 years, further highlighting the critical need for a practical, cost-effective solution to harmful autoimmune responses.
Through its focus on the COVID-19 testing market with AditxtScore™, Aditxt demonstrated the wide-ranging potential of its portfolio. Fortune Business Insights estimated the global COVID-19 diagnostics market at $48.64 billion for 2022. While demand for COVID-19 diagnostics is expected to lessen in the coming years, Aditxt will be uniquely positioned to leverage its existing infrastructure stemming from these operations as the company works to advance broader applications for the AditxtScore™ platform.
Leadership Team
Amro Albanna is the Co-Founder, Chairman, and CEO of Aditxt. He has founded multiple startups to commercialize innovations in various industries, including healthcare, enterprise software, telecommunications, nano technology, consumer health, and biotech. Mr. Albanna has led numerous M&A and going-public transactions as a founder, co-founder, and senior executive.
Shahrokh Shabahang, D.D.S., MS, Ph.D., is the company’s Co-Founder, Chief Innovation Officer, and a member of its board. He brings to the team more than 20 years of experience in developing and commercializing life science technologies focused on product and clinical development in the fields of microbiology and immunology.
Corinne Pankovcin, CPA, MBA, is the President of Aditxt. Prior to joining Aditxt, Ms. Pankovcin served as CFO for several world class organizations, including Business Development Corporation of America, Blackrock Kelso Capital and AIG Capital Partners. In these roles, Ms. Pankovcin was responsible for executing portfolio investments and managing significant M&A transactions.
Thomas Farley is the Chief Financial Officer of Aditxt. From December 2015 to June 2020, Mr. Farley was the Controller and Treasurer of Business Development Corporation of America (“BDCA”), a publicly listed business development company. Prior thereto, from January 2011 to August 2015, Mr. Farley was the Senior Controller of Blackrock Capital Investment Corporation (NASDAQ: BKCC). Prior to joining BlackRock Capital Investment Corporation, Mr. Farley was a Senior Controller for PineBridge Investments Emerging Markets practice. Mr. Farley was also an Accounting Manager for Bessemer Venture Partners prior to his tenue at PineBridge. Mr. Farley began his career with PricewaterhouseCoopers LLP, from 1996 to 2001. Mr. Farley earned his B.S. in Accounting from Long Island University and is a Certified Public Accountant.
Rowena Albanna is the company’s Chief Operating Officer. Ms. Albanna has over two decades of experience in senior leadership roles for both technology startups and public companies. Ms. Albanna’s experience spans a wide variety of industries, including biotechnology, insect control, nanotechnology, consumer electronics, financials, telecommunications, e-commerce, online marketing, medical, and defense.
Matthew Shatzkes is the Chief Legal Officer and General Counsel of Aditxt. As a former partner at an AM Law 50 law firm, Mr. Shatzkes advised a wide variety of healthcare related entities, including biotech companies, on corporate, regulatory, and strategic business matters. Mr. Shatzkes will oversee all aspects of the legal functions at Aditxt, including, providing advice and counsel on governance, regulatory matters, strategic alliances, mergers and acquisitions, and commercial transactions.
Advanced Container Technologies Inc. (OTC: ACTX)
Advanced Container Technologies Inc. (OTC: ACTX) is in the business of selling and distributing self-contained, automated, indoor “micro-farms” called Grow Pods, along with related equipment and supplies. Additionally, the company designs and sells patented proprietary medical-grade plastic containers, known as the Medtainer®, that store and grind pharmaceuticals, herbs, teas and other solids or liquids.
ACTX is the leading distributor of Grow Pods. With a controlled environment, food and herbs can be grown without pesticides, harmful chemicals or risk of pathogen contamination, and with low energy consumption. Restaurants, grocery stores, non-profits, MSOs and entrepreneurs can use Grow Pods to ensure a fresh supply of ultra-clean produce year-round.
The company entered the Grow Pod business in October 2020 with its acquisition of all shares of Advanced Container Technologies Inc., a California corporation. As of February 28, 2022, ACTX is exploring the acquisition of the assets and the assumption of some or all of the liabilities of GP Solutions Inc., the developer and manufacturer of Grow Pods, for which ACTX is currently the sole U.S. distributor.
Because Grow Pods can be located almost anywhere, produce can be grown closer to the point of consumption and harvested at its peak, providing nutritious fruits and vegetables where needed. Indoor micro-farms, utilizing a practice known as vertical farming, have attracted the attention of governments and universities, which are now promoting vertical farming as a way to combat food insecurity and inequities.
The United States Department of Agriculture (USDA) has stated that vertical farming “is no longer a futuristic concept.” The department is enthusiastic about vertical farming, particularly those utilizing repurposed shipping containers, such as Grow Pods. Arizona State University reports that vertical farming reduces water use by 90 percent compared to conventional farming but produces 10 times the crop yield.
Products
Grow Pods
One of the company’s main business units is focused on selling advanced, self-contained hydroponic containers called Grow Pods. These unique and innovative automated systems are essentially micro-farms that can be placed virtually anywhere and, with their controlled and specially filtered environment, allow cultivation of a wide variety of crops, 365 days a year. The Grow Pod controlled environment offers major advantages for the production of high-value crops. The ability to grow year-round and the ability to cultivate in a smaller footprint using less water and power are some of the primary advantages of the system. Grow Pods offer constant temperature, humidity and airflow control, as well as automated watering and lighting schedules for optimal growth and minimal labor requirements, regardless of crop.
Containers
ACTX meets the needs of the pharmaceutical and medical markets, including the cannabis and hemp industries, with patented packaging systems. The company designs, customizes, brands and sells proprietary medical grade plastic containers that can store pharmaceuticals, herbs, teas and other solids or liquids, with a special built-in feature that can grind solids and shred herbs. The company’s flagship container product is the patented Medtainer®, a child resistant, medical-grade herb container and grinder that is water-tight, air-tight and smell proof. Packaging in the cannabis industry is critical, with numerous stringent regulations about how cannabis products must be packaged and labeled. ACTX also offers custom-branded, compliant vacuum seal bags and other retail container solutions.
Equipment and Supplies
ACTX markets and sells two principal products: Grow Pods, which are specially modified insulated shipping containers manufactured by GP Solutions Inc., in which plants, herbs and spices may be grown hydroponically in a controlled environment, and Medtainers®, which may be used to store pharmaceuticals, herbs, teas and other solids or liquids and can grind solids and shred herbs. The company also markets and sells various products related to Grow Pods and the Medtainer®, as well as providing private labeling and branding services for purchasers of Medtainers® and certain related products.
GP Solutions manufactures and sells other products, such as humidity controllers and LED lighting systems for vertical farming. The company’s specially designed lighting panels are programmed to emit the exact wavelength of light that each crop requires. The system has a daybreak-to-nightfall feature that gives plants the proper chromatic signals to grow rapidly and fruitfully. High efficiency LED light strips supply the crops with a red and blue light spectrum required for photosynthesis in the spectrum that plants need most.
Market Overview
The global vertical farming market is expected to reach $33.02 billion by 2030, according to a new report by Grand View Research. The market is forecast to expand at a CAGR of 25.5 percent from 2022 to 2030, according to Grand View. Escalating production of biopharmaceutical products, including cannabis, is anticipated to drive the market. The building-based segment of the market is expected to register a significant CAGR of 27.8 percent over the projected period. In addition, the climate control segment is expected to see high growth.
The global cannabis packaging market is expected to reach $14.34 billion by 2028, according to analysis by Reports and Data. The analysis forecasts 1,700 percent growth in cannabis users by the end of 2026, with packaging likely observing a whopping 26.42 percent growth in the forecast period. There are significant barriers to entry in the cannabis packaging market, giving an advantage to companies already established in the sector. These barriers include developing a thorough knowledge of the myriad regulations that govern cannabis packaging (which differ in each state), and child-resistance requirements.
Management Team
Douglas P. Heldoorn is the Founder and Chairman of Advanced Container Technologies Inc. He also holds the positions of President, CEO and COO at the company. Mr. Heldoorn has served on the Board of Directors since its inception in 2013. He has also previously held the position of Executive General Manager at Nissan Motor Corp.
Jeffory A. Carlson is CFO and Treasurer of ACTX. Mr. Carlson has also served as the company’s Corporate Controller since 2014.
American Cannabis Partners

American Cannabis Partners (ACP) is a multi-state cannabis company with 560,000 square feet of licensed canopy space for cultivation and one retail license. The company is nationally headquartered in Trinity County of Northern California’s Emerald Triangle.
ACP is focused on three complementary business segments: real estate, acquisition & development of proprietary assets, and ongoing cultivation operations. Led by a seasoned management team with 30+ years of canna-business experience, ACP’s strategy is to capture opportunities in real estate and licensing in states that have recently passed cannabis legalization legislation, thereby equipping the company to capitalize on Federal interstate commerce opportunities.

Through its current cultivation operations, ACP supplies approximately 80% of its whole flower products for manufacturing, distribution and retail licenses. With the remaining 20%, the company supplies its proprietary strains to select California distributors and its own Michigan retail location under its exclusive in-house brand, ZÜK.
History of American Cannabis Partners
In 2014, Stephen Jordan, President of ACP, took on the Director of Operations position for a U.S.-based company operating in the Jamaican cannabis space. Over the course of his three-year tenure in this role, Jordan developed a number of relationships that would help serve as the basis of American Cannabis Partners.
One such relationship was with Junior Gordon, a cultivation lead grower from Jamaica’s Westmoreland Parish. Jordan immediately saw the value of Gordon’s unique skillset and credentials, and Gordon recognized Jordan’s heartfelt vision of bringing Jamaican culture to the rapidly developing U.S. cannabis space.
Guided by that mission, ACP’s unchanging goal is to improve the lives of individuals through cannabis and business.
Current Operations
Since its founding in 2018, privately-owned American Cannabis Partners has established a foothold in two key U.S. cannabis markets – California and Michigan. In total, the company has acquired 12 cannabis licenses, including 20,000 sq. ft. of cultivation licenses in California and 540,000 sq. ft. of cultivation licenses & one retail license in Michigan.
ACP’s IP portfolio features three proprietary strains sold exclusively through the company’s wholly owned ZÜK brand, as well as proprietary data collection and mining systems supporting its cultivation and retail operations.
Plans for Expansion
American Cannabis Partners is pursuing additional growth in the cannabis sector through multiple planned initiatives. These include:
- Submitting applications for additional cultivation licenses at the company’s Trinity County, California, location;
- Planning land acquisition and project development strategies for expanding operations to its third U.S. state beginning in the second quarter of 2022; and
- Planning land acquisition and project development strategies for expanding operations to its fourth U.S. state beginning in the second quarter of 2024.
ACP is currently exploring expansion opportunities through partnerships and joint ventures in New Jersey, New York, Virginia, Nevada, Arizona, Missouri and Massachusetts.
Management Team
Stephen Jordan is the President of American Cannabis Partners. He is focused on the first and last steps of legal cannabis – cultivation and retail. To date, Mr. Jordan has provided the company with ownership of 12 licenses, three proprietary cannabis strains and multiple real estate assets. His background in cannabis operations and financial strategies has guided American Cannabis Partners’ efforts to produce consistently high-quality product for both the medical and recreational segments. Mr. Jordan has operated under cultivation, manufacturing, distribution, medical research (Univ. of West Indies), retail and exportation licenses in multiple countries, further strengthening his network within the cannabis industry.
Gary Coltek is the company’s Director of Operations. He has credentials based in the culinary, hospitality and sustainability industries spanning over 40 years, including taking three companies public. Mr. Coltek has held management positions internationally with Ritz Carlton, Four Seasons, Trump Hospitality, Phymatrix and International Oncology Network. For 17 years, he was the founding member and partner of a private boutique consulting firm. He is currently a guest speaker and visiting professor at universities in Israel, China, Italy, the Netherlands and Peru, covering topics that include culinary sustainability, sustainable cannabis farming, organic sustainable farming and cannabis clinical studies.
Scot C. Crow is the Lead Corporate Counsel for American Cannabis Partners. He has extensive experience in corporate mergers & acquisitions and tax law. His clients rely on him to advise them with respect to their complex financial transactions and provide outside general counsel. Mr. Crow provides his clients proactive advice with respect to sensitive management matters, litigation management, day to day transactional needs and objective assessments for the development of successful business strategies. His experience includes serving as lead counsel for numerous mergers & acquisitions, private equity investments, private offerings, venture capital financings, mezzanine debt offerings, divestures and other related transactions, with an emphasis in the legalized marijuana segment.
Jacob Frenkel is the company’s Lead Compliance Counsel. He is the current Chair of Dickinson Wright’s Government Investigations and Securities Enforcement Practice. Mr. Frenkel’s solutions-minded approach to issues has earned him a reputation as an aggressive, tenacious, creative and proactive defense lawyer and litigator. After 14 years as a Senior Counsel in the SEC’s Division of Enforcement, U.S. federal criminal prosecutor and New Orleans Assistant District Attorney, Mr. Frenkel has practiced in the private sector for 20 years. His unique mix of corporate transactional, litigation and investigations defense clients extend well beyond the cannabis industry and cover a wide range of industries worldwide.
Junior Gordon is the Director of Cultivation for American Cannabis Partners. With 30 years of international cannabis cultivation experience in both the Caribbean and United States, Mr. Gordon is recognized as one of the top growers in the world. His skills span both controlled indoor and large volume outdoor harvest programs, giving him proficiency in nursery, propagation and indoor & outdoor grow strategies. As a winner of High Times and other notable Cannabis Cups, his focus is on connecting the dots between propagation, soil, irrigation, planting, harvesting, curing, processing and inventory control, bringing Jamaican cannabis cultivation best practices to American Cannabis Partners’ operations.
Amesite Inc. (NASDAQ: AMST)
Amesite Inc. (NASDAQ: AMST) is a high-tech artificial intelligence software company offering a cloud-based platform and content creation services for K-12, college, university and business education and upskilling. Amesite’s platform enables every business, institution and school to launch outstanding online learning programs, branded-to-them, in just days: easily, affordably and effectively.
Amesite-offered courses and programs are branded to its customers. The company’s customers gain mass customized environments for their learners, through cutting-edge, disruptive technologies and services, as well as patent-pending features. With over 98% retention of learners across all of its programs, Amesite gives customers an online solution for delivering learning that works!
With no institution-wide training required, Amesite launches learning programs in just weeks or days. The platform’s ease of use is unparalleled – and Amesite offers full training and customer support for customers and users! Signup is simple, and Amesite also integrates 1,000s of APIs, making sure that the solution works with existing tools and scales to customer needs.
Amesite uses artificial intelligence technologies to provide customized environments and greater accessibility for learners and easy-to-manage interfaces for instructors, in the U.S. education market and beyond. Amesite adheres to a business model that combines scalability and flexibility, positioning the company for growth in three main expanding markets: employee learning and development, K-12 and higher education.
Customized Solutions for Learning Communities
The company provides its services via a cloud-based, single-threaded, single page application that easily integrates with existing educational tools and systems while also enabling integration of best-in-class third party tools and custom-built features. In addition, the platform offers end-to-end security, as all hosting is handled by Amesite and any collected data is only used to improve learning.
Business Solutions
Through its customized and scalable online learning products delivered via a single, easy-to-use platform for both compliance and training, Amesite enables businesses to quickly and efficiently improve team performance.
Amesite’s products were designed to identify and address key issues in professional development learning, allowing customers to efficiently identify skill gaps, complete training and certification, and drive employee engagement, with the end goal of increased preparedness and productivity.
On signup, Amesite customers receive access to a branded platform equipped with all the learning products and programs their teams need, access to on-demand and customized content and instructors, and regularly updated employee learning programs. The product includes key features such as:
- Data analytics – Analysis of employee training progress and course correction insight, as well as detailed analytics at every level, helping management make informed decisions while upskilling their teams
- Customizable dashboards – Access to first-class communication tools with messaging and video conferencing capabilities, allowing clients to monitor employee learning progress
- Rapid launch within 24 hours – Fast launch and implementation of a system that meets employee needs, without compromising substance or quality and without requiring prior training
- Ease of use – A social media-inspired interface to keep employees and management engaged and reduce training time
Solutions for Universities and K-12 Schools
Amesite enables K-12 schools and universities to launch scalable and fully customized online educational products for every learner, without requiring additional staff or IT assistance. The company’s fully managed learning and upskilling platform is easy to use, requiring no training before deployment, while being equipped with a social media-inspired design to attract students and keep them engaged, and delivering courses and content with best-in-class compliance and security.
In addition, the platform is provided with customized courses that are in high demand and are continuously updated. It also tracks progress, maintaining a higher level of engagement and commitment to learning. Colleges and universities have access to expert curation, content and instructors, with state-of-the-art customer service, and they are able to pay as they go, only for the programs they need, fully branded to them.
As with the business solutions, K-12 schools and universities gain access to a series of helpful features and functionalities on signup with Amesite, such as launch within 24 hours, customizable dashboards, data analytics, ease of use and high engagement. Other key features include customizable grade books and automation, optimizing courses to learners’ needs and providing auto-graded assessments.
Solutions for Offices During COVID-19
Committed to helping businesses return to work during and after the COVID-19 pandemic, Amesite’s platform and features can help solve key problems organizations will face in the process.
The company offers turn-key technological solutions that allow businesses to bring their teams back to work safely and effectively. Moreover, Amesite can help leaders make decisions on returning to work by leveraging real-time, actionable analytics and tracking and analyzing employee data management in full compliance and with proven security.
Market Outlook and Opportunities
By combining AI-driven technologies with significant competitive advantages in terms of cost, engagement, results, scalability and flexibility, Amesite is uniquely positioned for fast growth in three core markets.
The employee learning and development sector in North America was valued at approximately $169 billion in 2019, driven by technological advancements in the field and a heightened focus on employee upskilling and professional development in the marketplace (https://ibn.fm/0ISNg).
The U.S. higher education sector and the K-12 segment are also fast expanding, providing multiple opportunities to Amesite and its turn-key educational solutions. There were approximately 16.6 million learners in the U.S. higher education system in 2018, following a steady increase in the number of undergraduate enrollments, while the K-12 sector had approximately 56.6 million students in 2019.
Corporate Awards
Amesite has been recognized by multiple organizations for its commitment to excellence, both in terms of its technology and its workplace culture.
The company’s recent accolades from The National Association of Business Resources, which focus on workplace excellence, include:
- 2020 Best and Brightest Companies to Work for in the Nation
- 2020 National Best and Brightest in Wellness
Additionally, Amesite’s cloud-based platform won Business Intelligence Group’s 2020 BIG Innovation Award, which recognizes organizations, products and people that are bringing new ideas to life in innovative ways.
Management Team
Ann Marie Sastry, Ph.D., is the President, CEO, Chair of the Board and Interim Chief Financial Officer of Amesite. Between 2008 and 2015, Dr. Sastry served as president, CEO, co-founder and member of the board of Sakti3, which was sold to Dyson Ltd. for $90 million. She continued with Dyson as head of the global solid state battery team, where she focused on technology advancement and strategy, organizational growth and partnerships. Before becoming an entrepreneur, Dr. Sastry was a professor of engineering at the University of Michigan, obtaining some of the highest honors in her scientific field throughout her 17-year scientific career. She holds Ph.D. and MS degrees from Cornell University and a BS in mechanical engineering from the University of Delaware. Her work has been featured in reputable publications such as Forbes, The Economist, USA Today, The New York Times, Wall Street Journal and many more.
Mike Smiley serves as Amesite’s Director of People. Mr. Smiley previously served as HR and administrative manager at Sakti3, where he received six workplace excellence awards. In this role, he helped integrate the Sakti3 team into Dyson following its acquisition, and then served as a member of Dyson’s People Enablement Team to help enable a high-performance culture via process improvements and organizational change. Mr. Smiley’s expertise encompasses performance management, global hiring and global HR collaboration on issues such as benefits, compliance and regulatory change.
AnPac Bio-Medical Science Co. Ltd. (NASDAQ: ANPC)
AnPac Bio-Medical Science Co. Ltd. (NASDAQ: ANPC) is a biotechnology company focused on early cancer screening and detection. The company develops, distributes and deploys accessible early disease detection devices with an aim of changing the way people approach cancer screening. AnPac Bio-Medical is a highly innovative company and an early thought leader and developer of multi-cancer screening technology, which is gaining significant acceptance.
AnPac Bio-Medical has clinical laboratories in the United States and China, with 142 issued patents as of March 31, 2021. Its corporate headquarters is located in Shanghai, China, while its U.S. headquarters is situated in Philadelphia, Pennsylvania. The company operates two certified clinical laboratories in China and one CLIA registered clinical laboratory in the United States.
Cancer Differentiation Analysis (CDA)
Cancer Differentiation Analysis (CDA) is AnPac Bio-Medical’s approach to detecting cancer and pre-cancerous diseases. CDA uses the natural biophysical properties of blood and cellular proteins to discover cancerous environments before the tumors even form.
Most liquid-based cancer screening and detection technologies focus on biochemical signals, like conventional biomarkers and genomic signals, such as ct-DNAs and CTCs (circulating tumor cells in the blood). These typically only determine whether or not cancer has occurred at a fixed point in time.
CDA technology combines an assessment of existing biomarkers with the biophysical properties and cellular proteins that signal the lead-up to serious health conditions and cancer. It is also used to pinpoint where cancer is most likely located and predict where the risk is highest in the future – all through a standard blood test, at a competitive price point.
AnPac Bio-Medical’s CDA is powered by a database of over 200,000 samples and cases and serves as a new way to approach disease and cancer screening. The device uses an integrated system of sensors to detect several biophysical signals at the cellular, protein and molecular levels. CDA leverages a proprietary algorithm to synthesize the data, effectively generating a personalized risk assessment for evaluated patients.
Through CDA technology, AnPac Bio-Medical aims to address a number of goals, including:
- Innovate – AnPac Bio-Medical is an innovator in the cancer screening industry, with CDA research ongoing since 2008, and commercial operations beginning in 2015. AnPac considers itself a thought leader in developing multi-cancer screening.
- Detect – AnPac Bio-Medical detects early signals of threatening cancer and its location within the body.
- Identify – CDA identifies the risks of up to 26 different types of cancers with high sensitivity and specificity rates.
- Provide – The company’s platform provides multi-level, multi-parameter analysis using proprietary diagnostic algorithms, which results in accurate and easy-to-understand results.
- Proven – A fully operational analysis of over 200,000 test samples has been run to date. CDA technology has been shown to identify pre- and early-stage cancers in patients previously diagnosed as “cancer-free” through traditional methods.
- Biophysical Properties – CDA analyzes biophysical properties in human blood and the correlation between biophysical properties and cancer occurrence.
Market Outlook
AnPac Bio-Medical is exploring detection of other types of cancers leveraging its innovative CDA technology and multi-cancer screening and detection tests, which could open significant opportunities on the global cancer diagnostics market.
According to a report by Grand View Research, the cancer diagnostics market is expected to reach $249.6 billion worldwide by 2026 (https://nnw.fm/L7css). The market is expected to grow at a CAGR of 7% during the forecast period.
Management Team
Dr. Chris Yu is the Co-Founder and Chief Executive Officer of AnPac Bio-Medical. He has enjoyed a successful career as an innovator in life sciences, technology and engineering. Dr. Yu has worked for three U.S. Fortune 500 companies and is the first/principal inventor of over 300 patent applications spanning semiconductors, materials and life science. He has a proven history of developing cutting-edge products with long-term profit and sustainability. Dr. Yu was born to a medical doctor’s family and went to medical school. He later switched his major to physics and received his bachelor’s and master’s degrees in physics from the University of Missouri-Kansas City Campus and a doctoral degree in physics from Pennsylvania State University. Both of his dissertations addressed innovative detection techniques.
Dr. Herbert Yu is the Co-Founder and Chief Medical Officer of AnPac Bio-Medical. He is a renowned expert in molecular epidemiology, with training in medicine and chemical biochemistry. Dr. Yu has a 20-year career in leading-edge cancer research, including breakthrough work in areas of carcinogenic factors. He is a professor and research director at the University of Hawaii and an adjunct professor at Yale University. He received his bachelor’s degree in medicine from Shanghai First Medical College. Dr. Yu also received a science degree in epidemiology and a Ph.D. in clinical biochemistry from the University of Toronto.
Jingiu (Edward) Tang is the company’s Chief Financial Officer. He previously served as a global internal auditor at Natuzzi S.p.A. Mr. Tang also worked at Beijing Dongshen CPA and Shanghai De’an CPA, providing external audits, finance and tax advisory services across different industries and sectors. He is a Certified Public Accountant in Australia. Mr. Tang received his bachelor’s degree in accounting from Charles Sturt University in Australia, his MBA from Charles Sturt University, and his bachelor’s degree in law from Southwest University of Science and Technology in China.
Weidong Dai is the company’s China General Manager. He previously served as a general partner at Stirrfir Investment Management Co. Mr. Dai has also served as the chairman of RTS Management (Shanghai) Co., and as managing director of Hong Kong Pro-Health Technology Co. and Shanghai Pro-Health Medical Devices Co. He has published a number of medical research papers and research articles in professional journals. Mr. Dai was awarded the Hong Kong Industrial Award for a medical device that he led in research and development. He earned his bachelor’s degree in medicine from Anhui Medical University, a master’s degree in medicine from the Sun Yat-San University of Medicine, and an Advanced Certificate of the EMBA CEO Program from Fudan University, School of Economics.
AREV Life Sciences Global Corp. (CSE: AREV) (OTC: AREVF)
AREV Life Sciences Global Corp. (CSE: AREV) (OTC: AREVF) is a fully integrated, publicly traded, early-stage life science enterprise dedicated to delivering therapeutic interventions to public health through discovery, innovation and successful collaborations in the life science industry. The company’s leadership drives discovery programs for clinical complexities presented by malnutrition, viral infectious diseases and the inflammatory response system.
AREV’s business model leverages the core competency of producing proprietary compounds through its innovative extraction methodologies, scientific advisory board (SAB), experienced staff, and executive leadership to drive its product pipeline. AREV’s strategy is to generate revenue from selling its branded products via its online technology platform, Medicine Merchant™, an enterprise marketing platform built to enable consumers to have access to novel therapeutic approaches to human nutrition, endemic diseases, and neglected chronic related co-morbidities. The company also expects to generate revenue from toll processing and government procurement of its products that address malnutrition and related health issues.
AREV is focused on innovations in biomedicine and maintains a significant footprint in clinical human nutrition utilizing proprietary protein blends and terpenes, complimented with vitamins and minerals. The company has utilized its expertise to design and deliver innovation in therapeutic interventions using its exclusive botanical, fungi and marine compounds to address medical conditions driven by presenting global epidemiological characteristics of multiple challenges to international human and animal health. AREV uses its proprietary extractions allowing characterizations from botanical and marine sources in therapeutic foods and medicines that comprise its development pipeline.
Development of AREV’s pipeline is fostered by collaborations with academic centers, clinical research organizations and government institutions committed to facilitating discovery of promising new clinical approaches presented in peer reviewed journals. AREV operates under the guidance of its SAB and a growing number of strategic collaborations with CROs and academic research centers, including the Linus Pauling Institute at Oregon State University.
AREV is a member of both BIOTECanada and The Biotechnology Industry Organization (BIO):
- BIOTECanada is the national industry association with over 200 members located nationwide, reflecting the diverse nature of Canada’s health, industrial and agricultural biotechnology sectors.
- BIO is the world’s largest advocacy association representing member companies, state biotechnology groups, academic and research institutions, and related organizations across the United States and 30+ countries.
Products
AREV’s end-product and target categories include therapeutic interventions, botanical drugs, ready-to-use therapeutic food (RUTF), enteral nutrition formulas and early-stage small molecule antiviral therapeutics demonstrating novel mechanisms of action.
Wright and Well Branded Line
Wright and Well Branded Line is the company’s branded line of therapeutic cannabinoid and terpene-based formulations. The topical line includes solutions for burns, wounds, skin disorders and muscle relief, as well as a lubricant for intimacy. The oral line addresses inflammation, heart health, high blood pressure and viral infections. These products are currently produced at CBD99, a licensed processor in Sandy, Oregon. The company also has a strategic relationship with a Canadian licensed hemp and cannabis processor in Vancouver, British Columbia.
SUS-TAINN™
The company’s products in the RUTF category are branded under SUS-TAINN™ (Superior Utility Supplementation Therapeutic Agent for Indicated Nutritional Needs). SUS-TAINN™ is the flagship line of products stemming from AREV’s collaboration with Voynich Biosciences.
RUTFs like SUS-TAINN™ are the cornerstone to international famine response and currently represent more than 20 percent of public health commodity procurement spending. SUS-TAINN™ is purchased by agencies ranging from the U.S. Department of State AID for International Development to the World Food Program and is distributed by an increasingly substantial number of non-governmental organizations.
RESTORE™
The company’s enteral nutrition product is branded as RESTORE™. For patients experiencing caloric compromise, enteral nutrition, a liquid form of nourishment, is often required. RESTORE™, the initial enteral formulation from AREV, is based on organic plant nutrition for patient populations who have demonstrated overt clinical need for rational enhanced caloric intake and micronutrient supplementation.
RESTORE™ provides a proprietary blend of high-quality proteins, antioxidants, minerals and proven anabolic agents, combined with pre- and probiotics. The company plans to submit for Medicaid formulary inclusion and reimbursement designations in various jurisdictions characterized by the Ryan White CARE Act during the second half of 2022.
REV-I1™
REV-I1™ is the company’s small molecule drug discovery model for phytomedicinalization, based on advanced computational characterization and next generation affinity selection that affords new opportunities in HIV antiretroviral research. REV inhibition offers a critical approach to inhibiting HIV replication and addressing viremia in highly conserved sanctuary regions.
Phytomedicinal therapeutic discovery and development offer clinically viable approaches to a wide range of scientific challenges that currently elude successful contemporary interventions. A cornucopia of diverse validated compounds allows AREV to explore a distinguished range of promising approaches that are in stages of pre-clinical validation. Combining AREV’s versatile extraction capability with highly sensitive analytical techniques (LC-MS, MS-MS) is expected to allow new medicinal chemistry to be identified and characterized, leading to therapeutic candidates in the company’s commercial drug discovery platform.
Market Overview
A report from Verified Market Research valued the global plant protein market at $29.4 billion in 2020 and forecast that it could surpass $162 billion by 2030, which would make up 7.7% of the global protein market, according to a report released in August by Bloomberg Intelligence.
The RUTF market was valued at $363.72 million in 2019 and is projected to reach $807.89 million by 2027, growing at a CAGR of 10.5% for the forecast period. The market is primarily driven by the efforts of governments and non-governmental organizations to reduce the rate of severe acute malnutrition. Moreover, changing trends and growing developments related to diet-related deficiencies, as well as increasing incidences of famine and disasters, are likely to fuel the growth of the RUTF market in the near future, according to the report.
Management Team
Michael Withrow is the founder, chairman and CEO of AREV. He is a successful natural products and technology entrepreneur with more than 25 years of experience. He has started and sold companies and has worked with companies such as CAVA Health Care (formerly Alternative Extracts Inc.), North American BioExtracts Inc., and Canadian Pacific Phytoplankton Ltd. He has also served as president and CEO of a specialty cannabis technology company.
Kevin Phelps, CPA, is a director at AREV. He is CEO and president of Immune Therapeutics Inc., a bio-pharmaceutical company. He began his career with Price Waterhouse before joining Eastman Kodak Company as part of an executive team that successfully spun out the Bio Products Division into Genencor International Inc., an international industrial bio-chemicals company. He later joined Trillium Group, a regional private equity firm, as a partner, where he served on behalf of the firm as CFO of Vaccinex Inc., a vaccine development company, and chairman of AccuMed Inc., a medical device company. He has served as Chairman of Oyagen, a biotech that over the past decade has developed drug discovery methods that have enabled it to explore vulnerabilities in HIV, Ebola and coronaviruses.
Denby Greenslade is corporate secretary and interim CFO at AREV. She has served as corporate secretary and director for several companies in the mining, biotech and IT industries and has more than 15 years of corporate secretarial, corporate governance, and securities regulation experience. She graduated from Simon Fraser University with a Bachelor of Arts in Communication.
Allan Echino is a director at AREV. He was a founder and director of Corlac Resources, an oil producer, and Calroc Industries, an oil service company based in Alberta. Mr. Echino arranged funding for a licensed producer in the cannabis sector and quickly learned the business and health benefits of cannabis and mushrooms.
Mel Maxwell is a director at AREV. During his 40-plus year career, he has founded six companies. His degrees in business and computer science initially took his career toward IT ventures that entailed software development, IT consulting, e-commerce solutions, real estate development, pathogen remediation solutions and wholesale vehicle aggregation. He finds continued enjoyment in consulting with other businesses and entrepreneurs.
Scientific Advisory Board
Roscoe M. Moore, Jr., DVM, MPH, PhD, a former United States Assistant Surgeon General, provides strategic planning for AREV’s nutritional and drug discovery platforms as chairman of the company’s scientific advisory board. Dr. Moore is a member of the board of advisers of the Institute of Human Virology (IHV) and the board of directors of the Global Virus Network associated with IHV, University of Maryland Medical Center. IHV is the first research institute in the U.S. to link basic science, population studies, and clinical trials in an effort to develop new vaccines and treatments; its 80 faculty members contribute to research on pandemic pathogens, ranging from COVID-19 to HIV.
Dr. Moore served with the United States Department of Health and Human Services (HHS) and was responsible, for the last 12 years of his career, for global development support within the Office of the Secretary, HHS, with primary emphasis on implementing innovations in essential health care commodity procurement programs for resource-challenged countries. Dr. Moore was a career officer within the Commissioned Corps of the United States Public Health Service, entering with the U.S. National Institutes of Health (NIH) and rising to the rank of Rear Admiral. Dr. Moore worked at the Center for Veterinary Medicine, U.S. Food and Drug Administration (FDA), before becoming senior epidemiologist within the National Institute for Occupational Safety and Health of the U.S. Centers for Disease Control and Prevention (CDC), where he also served as an epidemic intelligence service officer.
Robert Melamede, PhD, received his doctorate in molecular genetics and biochemistry from the University of the City of New York Graduate Center, focusing on base excision repair of free radical damages in DNA. For decades, he led laboratory efforts in a world-class, federally funded lab, where he discovered endonuclease VIII. Dr. Melamede did a sabbatical at the Scripps Institute and subsequently established an in vitro monoclonal antibody facility at the University of Vermont, developing antibodies to free radical damages in DNA and to DNA repair enzymes. Working with a collaborator, Dr. Melamede worked on metabolism and cancer cells as a professor and chair of the biology department at the University of Colorado at Colorado Springs. He co-founded Cannabis Science Inc., a public company, retiring as the company’s chief executive in 2014. Dr. Melamede continues his innovative research in the private sector.
Harold Smith, PhD, is the founder, CEO, and president of Oyagen Inc., a biotechnology company developing therapeutics for various disease states, including HIV and cancer. Dr. Smith is also a tenured professor of biochemistry and biophysics at the University of Rochester, School of Dentistry and Medicine, with additional appointments as a professor in genetics and pathology and as a member of the Center for RNA Biology.
Richard Van Breemen, PhD, is a Professor of Medicinal Chemistry in the Department of Pharmaceutical Sciences of the College of Pharmacy at Oregon State University. His research interests include cancer prevention by dietary antioxidants and prostate cancer prevention by the carotenoid lycopene, mass spectrometry-based pharmacological screening of natural products and combinatorial libraries for drug discovery, and high throughput screening to assess drug metabolism, toxicity and bioavailability. Dr. Van Breemen made The Analytical Scientist’s Power List in 2020 and 2021. His work in biomedical mass spectrometry earned him an initial spot, and he clocked in the Top 100 analytical scientists from around the world on the Power List in 2021.
Dr. Blake Hawley is the founder & CEO of Motega Health Inc. Dr. Hawley has worked in animal health, pharma and food across 23 countries and four continents. He holds an MBA from KU and his doctorate in veterinary medicine and zoology degrees from NC State. He serves on multiple non-profit advisory boards, including the KU MBA Advisory Board. Dr. Hawley is a 2017 graduate of Pipeline Entrepreneurs and a graduate of the Village Capital Agricultural 2016 Entrepreneur Cohort.
Dr. Hawley previously served as the General Manager of Australasia; Regional General Manager of Russia and Central Eastern Europe (consisting of 22 countries); and Managing Director of the United Kingdom and Ireland for Hill’s Pet Nutrition, a division of Colgate-Palmolive. His experience includes 10 years of profit and loss responsibilities in these territories, with consistent double-digit annual revenue growth in each of the 10 years. He oversaw products competing in the arthritis, dermatology, obesity, gastrointestinal, urinary, and cancer markets, among others. Dr. Hawley also has background in e-commerce, data analytics, and social media and most recently served as Worldwide Director of Global Digital for Hill’s Pet Nutrition.
Uma Dhanabalan, MD, MPH, FAAFP, MRO, CMS, advises AREV on product development and formulation. She is the founder and CEO of Global Health & Hygiene Solutions LLC, whose mission is to promote wellness and prevent illness locally and globally and runs an independent practice at Uplifting Health & Wellness in Cambridge, Massachusetts. Dr. Dhanabalan, a fellow of the American Academy of Family Physicians, graduated from UMDNJ, Newark, New Jersey, with her medical degree and trained in family medicine at MUSC in Charleston, South Carolina; she earned her master’s degree in public health from Harvard’s School of Public Health in Boston, Massachusetts, and continued her training at Harvard in occupational and environmental medicine.
Special IP Advisor
Douglas Sorroco practices in all areas of intellectual property law including patent, trademark, copyright, technology, and e-commerce and assists clients with intellectual property matters requiring litigation, licensing, technology counselling and complex transactions.
Mr. Sorroco is ranked in Band 1 (the top band) for intellectual property law by the highly regarded Chambers USA: America’s Leading Lawyers for Business 2021, and he was selected for inclusion in The Best Lawyers in America® 2022 for Litigation – Intellectual Property, Litigation – Patent, Patent Law and Technology Law. In 2021, Best Lawyers named him the Oklahoma Lawyer of the Year for Technology Law. In 2021, Managing Intellectual Property continued to rank Doug as an IP Star. Mr. Sorroco was selected for inclusion in Oklahoma Super Lawyers 2021 and was also selected by attorney peers for inclusion in Oklahoma Super Lawyers – Rising Stars Edition (2010).
Augmedix Inc. (NASDAQ: AUGX)
Augmedix Inc. (NASDAQ: AUGX), a leading provider of virtual medical documentation and live clinical support based in San Francisco, California, is on a mission to “rehumanize healthcare.” The company has set out to tackle the largest pain point in the U.S. healthcare system – the burden of documentation and administrative tasks on physicians.
Augmedix enables physicians to have natural interactions with patients without being distracted by taking notes during patient visits. When physicians are free to focus on just being physicians, they have been found to save up to 3 hours per day, improve productivity by as much as 20%, and increase satisfaction with work-life balance by over 40%. The time spent by physicians on documentation is estimated by management to cost the US healthcare industry as much as $100 billion annually and adversely impacts both physician and patient satisfaction.
By increasing physician satisfaction, Augmedix is also addressing a significant and growing problem in the U.S. healthcare industry – physician burnout – which costs the industry an estimated $4.8 billion every year. The cost to replace one physician can be as high as $1 million for a healthcare system.
Augmedix’s Platform and Technology
A physician’s exclusive focus on the patient is made possible by virtual medical documentation and live clinical support. Physicians are provided dedicated Google Glass units or smartphones with which they access the Augmedix service. As the physician and the patient interact naturally, the physician’s device enables the interaction to be observed remotely by trained medical documentation specialists who enter key inputs for the medical note. The company’s proprietary Ambient Automation Platform, incorporating structured data models, automatic speech recognition (ASR) and machine learning models, then takes those inputs and converts them into a medical note. This medical documentation is then uploaded into the patient’s electronic health record (EHR) for sign off by the physician.
Augmedix pioneered the concept of remote, real-time medical note documentation. Its approach to clinical documentation enables not only natural physician-patient conversations, which are very important for patients and physicians, but also physician mobility, given the mobile devices through which the service is accessed and the virtual nature of the company’s offering.
Augmedix’s Two Levels of Service
The company’s service is designed for ease of use by physicians. Entering a unique authentication code, physicians open a secure communication channel to the service platform through a dedicated smartphone or Google Glass unit supplied by Augmedix. Physicians have a choice of real-time service or non-real time service.
Augmedix LIVE – Real-Time Service
Under its real-time service offering, called “Live,” Augmedix’s highly-trained specialists observe the interaction between the physician and the patient, both visually and audibly, and make the appropriate contextual selections within the proprietary note creation application, “Notebuilder”. This then drives the company’s auto sentence generation models within the Notebuilder tool. The completed medical note is then supplied back to the physician for review and sign-off at the conclusion of the patient encounter.
Augmedix NOTES – Non-Real Time Service
Through its non-real time service, which the company calls “Notes,” the conversation between the physician and patient is recorded. It then passes through the Augmedix ASR software model. The text output is edited by specialists and further processed by Notebuilder into a comprehensive and accurate medical note. Medical notes in the non-real time service are supplied back to the physician prior to the next shift, typically the next day.
Utilizing either service, the administrative burden is lifted from physicians, who no longer need to type notes into their EHR system, allowing them to focus their energy on what they are trained to do – taking care of patients.
Specialties and Health Systems
Augmedix is compatible with more than 35 specialties and trusted by four of the top 10 and six of the top 20 enterprises, as measured by patient revenue, and many physician group practices supporting medical offices, clinics, hospitals, and telemedicine across the U.S.
Augmedix and Telemedicine
In recognition of the challenges imposed by the COVID-19 pandemic on in-person patient visits, Augmedix services are compatible with telemedicine, which will likely remain a meaningful healthcare delivery platform well after the pandemic has passed. Importantly, Augmedix is application-agnostic, meaning that it can accommodate any application used by physicians for remote patient encounters.
Market Outlook
Augmedix’s service is provided under a monthly subscription revenue model. As such, the company maintains high visibility into its future business outlook. Augmedix’s Net Promoter Score of 56 is more than twice that of the healthcare industry average of 27 and is yet another testament to its value proposition.
Over time, the company aims to increase the automation of clinical documentation, which represents a multi-billion-dollar market opportunity. As more data is collected and harvested through machine learning, Augmedix’s models will be able to automatically process an increasing portion of the medical note across an increasing number of patient visit types. Automation benefits the company’s business by reducing the time to create the note, as well as providing more uniform note quality, among other operational benefits.
Augmedix estimates its total addressable market to be about 300,000 physicians in the U.S., representing a potential annual revenue opportunity of about $6 billion. The company has a direct expansion path to about $1 billion among the healthcare enterprises already under contract. But Augmedix won’t stop there. It is continuing to sign up new major accounts through its “land and expand” sales and marketing strategy.
Management Team
Emmanuel “Manny” Krakaris is the President, Chief Executive Officer and Secretary of Augmedix. Mr. Krakaris has been a member of the Augmedix board of directors since October 2018. Prior to Augmedix, Mr. Krakaris served as the Chief Executive Officer of Streetline Inc., from August 2014 to February 2018, and as Chief Financial Officer and Chief Operating Officer, from 2011 to August 2014. Mr. Krakaris also served as Chief Financial Officer of Command Audio Corporation from 1996 to 2011. Mr. Krakaris received a Bachelor of Commerce in Marketing and International Business from McGill University and an M.B.A. from the University of California, Berkeley, Haas School of Business.
Paul Ginocchio joined Augmedix in July 2020 as Chief Financial Officer. Prior to joining Augmedix, Mr. Ginocchio served as an independent strategic advisor to multiple technology companies. Mr. Ginocchio previously served as Chief Financial Officer of Brightfield Strategies LLC, a workplace data and analytics company, from January 2017 to September 2019. From November 1998 to May 2016, Mr. Ginocchio was Lead Analyst, then Managing Director of Information & Business Services Equity Research at Deutsche Bank AG. Mr. Ginocchio holds a B.A. in Economics & Business Management from North Carolina State University, and an M.B.A. in Finance from Indiana University Kelley School of Business.
Sandra Breber has served as Chief Operating Officer of Augmedix since March 2019 and, prior to that, served as an advisor to the company from November 2018 to March 2019. Ms. Breber also served as President and Co-founder of Ziploop Inc. from April 2013 to November 2018. Prior to that, she was a management partner at Andersen, a global professional services firm. Ms. Breber holds a Bachelor of Commerce in Accounting and Finance from McGill University.
Saurav Chatterjee joined Augmedix in November of 2020 as Chief Technology Officer. Prior to joining Augmedix, Mr. Chatterjee served as Vice President of Engineering at Lumiata Inc. Mr. Chatterjee also served as the Senior Director and Head Conversational AI at Asurion Inc., from May 2014 to October 2019. Mr. Chatterjee holds a B.A in Electrical Engineering and Computer Science from the University of California, Berkeley, and a PhD in Computer Engineering from Carnegie Mellon University.
Jonathan Hawkins joined Augmedix in April 2019 as its Chief Revenue Officer. Prior to joining Augmedix, Mr. Hawkins served as Senior Vice President of Business Development, Sales and Marketing for Spry Health Inc., a healthcare data analytics provider that identifies early signs of clinical deterioration in chronically ill patients. Mr. Hawkins was also a Founding Investor and Advisor to The Batchery, a startup incubator and accelerator. Prior to that, Mr. Hawkins was Vice President of Business Development and Sales for MedeAnalytics Inc. Mr. Hawkins holds a B.A. in International Relations from Stanford University and an M.B.A. from Harvard Business School.
Ian Shakil is the founder of Augmedix and has served as Chief Strategy Officer since October 2018. Mr. Shakil has been a member of the Augmedix board since April 2013. From April 2013 to October 2018, Mr. Shakil served as the Chief Executive Officer of Augmedix. Mr. Shakil has also served as Advisor to Edwards Lifesciences Corporation since May 2019, and as Advisor to Maya.com.bd since January 2018. Mr. Shakil has a B.S.E. in Biomedical Engineering from Duke University and an M.B.A. from Stanford University Graduate School of Business.
Avricore Health Inc. (TSX.V: AVCR) (OTCQB: AVCRF)
Avricore Health Inc. (TSX.V: AVCR) (OTCQB: AVCRF) is a pharmacy service innovator focused on acquiring and developing early-stage technologies aimed at moving pharmacy forward. Through its flagship offering, HealthTab™ (a wholly owned subsidiary), the company aims to make actionable health information more accessible to everyone by creating the world’s largest network of rapid testing devices in community pharmacies.
HealthTab
HealthTab is a turnkey point-of-care testing solution that effectively turns pharmacies into diagnostic hubs (sometimes known as ‘Community Diagnostic Centers’, or CDCs) and connects them on a single, cloud-based platform.
The HealthTab network model is unlike anything in pharmacy today. It gives knowledgeable and trusted pharmacists a greater role in primary care delivery and empowers patients to take more control of their health. It also reduces costs and waiting times while providing many potential revenue streams, including equipment leasing & consumables, direct access testing, disease prevention & management programs, sponsored health programs, decentralized clinical trials, real world data (RWD) sets and third-party app integration through API.
Agreement with Shoppers Drug Mart
In June 2021, Avricore signed a Master Agreement with select Shoppers Drug Mart pharmacies to pilot the HealthTab platform. This agreement gives patients access to point-of-care blood screening and health-data management for potential risks relating to diabetes and cardiovascular conditions using HealthTab-integrated Afinion 2™ analyzers provided by Abbott Rapid Diagnostics.
Avricore is the first pharmacy solutions provider to partner with Abbott (NYSE: ABT), the global health care company and diagnostics leader in Canada. In May 2021, the company signed a supplier distribution agreement to expand the distribution of Abbott’s Afinion 2 and associated tests for diabetes and heart disease screening in community pharmacies in Canada. This agreement includes valuable HbA1c testing, a critical marker for the screening and management of diabetes.
Near Term Goals
Near term goals for Avricore include expansion into more pharmacies across Canada, followed soon after by entering the U.S. and UK markets. The company has made significant strides in testing and developing its technology and is moving into the commercialization stage.
Strategic partnerships like those with Abbott and select Shoppers Drug Mart pharmacies advance Avricore closer to becoming an incredibly dominant player in the community diagnostics space. The company aims to make actionable health information more accessible for everyone by creating the world’s largest rapid testing network in pharmacies.
Market Outlook
In 2020, the global point-of-care testing (POCT) market was valued at $34.49 billion and expected to expand at a compound annual growth rate (CAGR) of 9.4 percent to reach a projected $81.37 billion by 2028. This upsurge is expected to be driven largely by increased demand for screening and management tools for chronic diseases, as well as rapidly assessing infectious diseases such as COVID-19.
The accessibility of POCT has been an increasing priority of the world’s leading health organizations and experts. Pharmacies are ideal ‘hubs’ within the community to offer patients better access to the numbers they need to know for preventing or treating conditions such as diabetes and heart disease or the timely diagnosis of infection.
Management Team
Avricore’s leadership team brings a diverse portfolio of expertise across the health care and biotech industries, as well as technology, finance and communications. Together, they share a common vision of moving pharmacy forward and have positioned the company for significant future growth and expansion.
Hector Bremner is the CEO of Avricore. He has over 15 years of senior and executive experience across various industries, including international trade, natural gas, marketing and communications. He owned and operated TOUCH Marketing, a boutique marketing and communications firm based in Vancouver, from 2007 to 2013. Mr. Bremner has also served as the executive assistant to the Deputy Premier and Minister of Natural Gas Development, Responsible for Housing, as well as the Minister of International Trade and Minister of Small Business. In 2015, he joined Vancouver’s Pace Group Communications as VP of Public Affairs.
David Hall is the Chairman and a Director of Avricore. His leadership spans five different companies. He is currently the Chairman of RepliCel Life Sciences and a member of the boards of TrichoScience Innovations, AdvantageBC and Providence Health Care Research Institute. Mr. Hall also served as Chairman of Perceptronix Medical Inc.; Chief Financial Officer, Secretary & Treasurer of Angiotech Pharmaceuticals Inc.; President & Director at Newcastle Resources Ltd.; and Chairman for LifeSciences British Columbia.
Kiki Smith is Avricore’s CFO. She has over 20 years of experience assisting private and public companies in the roles of accountant, corporate controller and CFO in mining, oil & gas, real estate, high technology, food production and investment fund management. She currently provides consulting services in M&A, financial reporting and regulatory compliance to several public and private companies across several investment sectors. Ms. Smith is a member of the Chartered Professional Accountants of British Columbia and has a bachelor’s degree in economics from the University of British Columbia.
Rodger Seccombe is the Head of Avricore’s HealthTab division and the co-founder and former CEO of HealthTab Inc. Mr. Seccombe has over 20 years of experience launching and running companies in software, health care technology and clean energy. He is a recognized industry expert in direct-to-consumer and point-of-care testing technology. In 2006, he joined the start-up team at Canadian Bioenergy Corporation and helped pioneer the development of the renewable fuel industry in Canada. Before HealthTab, he designed and developed cloud-based informatics systems currently in use by some of the world’s leading medical laboratories and instrument manufacturers.
BevCanna Enterprises Inc. (CSE: BEV) (OTCQB: BVNNF) (FSE: 7BC)
BevCanna Enterprises Inc. (CSE: BEV) (OTCQB: BVNNF) (FSE: 7BC) is a diversified health & wellness beverage and natural products company focused on developing and manufacturing a range of plant-based and cannabinoid beverages and supplements for both in-house brands and white-label clients. The BevCanna team boasts decades of experience creating, manufacturing and distributing iconic brands that resonate with consumers on a global scale.
BevCanna’s distribution network features more than 3,000 points of retail distribution through the company’s market-leading TRACE brand, its Pure Therapy natural health and wellness e-commerce platform, its fully licensed Canadian cannabis manufacturing and distribution network and its partnership with #1 U.S. cannabis beverage company Keef Brands.
Based in British Columbia, Canada, BevCanna was founded in 2017.
End-to-End Turnkey Beverage Manufacturing Solutions
BevCanna is a manufacturer of traditional and cannabis-infused beverage brands serving a growing roster of white-label clients, in addition to operating a portfolio of in-house and partner brands. The company offers a full-service white label beverage manufacturing solution.
- Processing – At its state-of-the-art beverage manufacturing facility, BevCanna partners with industry leaders specializing in crude extraction, refinement, purification and solubility conversion to provide high-quality water-immiscible emulsions that maximize bioavailability, clarity and taste.
- Spring Water – BevCanna directly owns a pristine naturally alkaline spring water aquifer in British Columbia.
- Product Development – BevCanna leverages its expertise to develop captivating flavors based on category and consumer insights in order to enhance product positioning.
- Packaging – A variety of packaging options are offered by BevCanna, including beverage and nutraceutical formats such as PET, aluminum and glass, available in a variety of standard and custom sizes and shapes.
- Beverage Manufacturing: Traditional & Cannabis Facilities – The company’s 40,000-square-foot beverage manufacturing facility is HACCP (Hazard Analysis Critical Control Point) Certified. The facility’s capabilities include blow molding, dosing, carbonation options, filling and capping, pressure sensitive and shrink-sleeve label applications, flash pasteurization, QA testing and packing/palletizing for shipment.
Pure Therapy, TRACE and Partner Brands
BevCanna’s in-house brands include Pure Therapy and TRACE.
Pure Therapy is a direct-to-consumer e-commerce brand that markets a range of natural health products, including nutraceuticals and hemp-based cannabidiol (CBD) products, throughout North America and Western Europe.
Pure Therapy has secured orders from over 23,000 customers since its inception in 2017. BevCanna expects strong growth through Pure Therapy over the next 12 months driven by new product integration, accelerated growth of existing products and its marketing team’s e-commerce expertise.
TRACE products feature the Naturo Group’s proprietary plant-based fulvic and humic mineral formula, sourced from deep within the Rocky Mountains of interior British Columbia. These unique and ancient minerals provide wellness properties that include iron, magnesium, calcium, potassium and many other minerals no longer found in our food chain at adequate levels.
Research suggests that the proprietary fulvic and humic organic compounds found in TRACE products could offer a number of key benefits, including promoting gut health, immune function, cognitive performance and whole-body wellness.
TRACE products include Natural Alkaline Spring Water, Plant-Based Mineralized Spring Water, Natural Flavor Sparkling Spring Water, Plant-Based Mineral Concentrate with Vitamin D and Plant-Based Mineralized Immune Support Shots.
In addition to its in-house brands, BevCanna provides white-label services to a number of partners in its space. BevCanna’s current portfolio of brand partnerships includes #1 U.S. cannabis beverage brand Keef (cannabis-infused classic soda) and BLOOM (live resin & high-end extracts). BevCanna also has multiple white label agreements to co-manufacture branded beverages.
Market Outlook for Cannabis-Infused Beverages
In 2018, the cannabis-infused beverage market was valued at $901.8 million. The market is expected to grow during the forecast period of 2019 to 2025 at a CAGR of 17.8%, resulting in a market value in excess of $2.84 billion by 2025, according to Grand View Research (https://ibn.fm/VkJfH).
The projected growth is largely attributed to the legalization of recreational and medical marijuana in multiple jurisdictions. Cannabis-infused beverages are uniquely positioned to provide an alternative to a large portion of the edibles market, including items such as chocolates, cookies, gummies and other types of confectionery pieces.
Management Team
Marcello Leone is the CEO and Founder of BevCanna. He is also the founder of Naturo Group and the TRACE brand.
John Campbell is the CFO and CSO of BevCanna. He has over 30 years of experience in the investment industry, including time with TriView Capital Ltd.
Keith Dolo is the company’s Executive Management Advisor, having previously served as CEO and Executive Chairman of Sproutly Inc. Previously, he served for over 13 years with Robert Half (NYSE: RHI), an S&P 500 company, specifically in the role of Vice President for the last eight years.
Melise Panetta is the company’s President. She is an accomplished senior marketing and sales executive with extensive experience leading organizations such as SC Johnson, General Mills (NYSE: GIS) and PepsiCo (NASDAQ: PEP). Ms. Panetta has nearly 15 years of deep marketing and sales expertise.
Raffael Kapusty is the company’s Vice President of Sales & Insights. She is an accomplished CPG industry leader with more than 25 years of experience in both the Canadian and U.S. retail spaces. With a solid foundation at ACNielsen Canada (NYSE: NLSN), Ms. Kapusty has developed a deep understanding of the CPG space, working with over 100 leading Canadian & global CPG manufacturers. She has also held senior category and key account management roles at Kroger (NYSE: KR), SC Johnson and Unilever Canada (NYSE: UL).
Bill Niarchos is the company’s Vice President of Sales & Sales Operations. He has over 20 years of experience in the CPG goods industry/retail environment. In his most recent role as Director of Sales with Bayer Consumer Health, Mr. Niarchos managed the strategic direction and growth of Loblaw & SDM. Prior to his position with Bayer (ETR: BAYN), Mr. Niarchos held a number of progressive roles at Colgate Palmolive (NYSE: CL) for more than 14 years.
Japheth Noah is the company’s Head of Quality Assurance. He is an Oxford and MIT educated quality and regulatory manager with over 15 years of experience in the beverage, pharmaceutical, natural health and medical industries.
Keith Stride is the company’s Creative Director. He has 25 years of experience in marketing and advertising, including time in a CMO role with Hemptown USA. Mr. Stride is internationally recognized for building high-profile brands, including Rogers (NYSE: RCI), TD Bank (NYSE: TD), Best Buy (NYSE: BBY), Whistler-Blackcomb and RBC (NYSE: RY).
Blue Hat Interactive Entertainment Technology (NASDAQ: BHAT)
Blue Hat Interactive Entertainment Technology (NASDAQ: BHAT) is a cutting-edge creator, developer and operator of popular augmented reality (“AR”) interactive smart toys and educational games in China. Blue Hat’s mobile-connected entertainment platform connects physical items to mobile devices through wireless technologies, creating a unique interactive user experience in various mobile games, interactive educational materials and toys with mobile game features.
Blue Hat designs original toys and games that utilize augmented reality technology, motion capture technology, image recognition technology, voice control, light sense technology, infrared, levitation induction, and other trending scientific technologies to transverse the virtual with reality. Blue Hat creates a rich visual and interactive environment for users through the integration of real objects and virtual scenery. This combination provides users with a more natural form of human computer interaction, enhances a user’s perception of reality, and delivers a more immersive entertainment experience.
Proprietary Technology
Founded in 2010, Blue Hat’s proprietary technology, product research and development, marketing channels and brand operation are the cornerstones of the business. Blue Hat focuses on the combination of “online” and “offline” activity and the interaction between “entertainment” and “product” to create a high-tech entertainment platform combining mobile games and AR. With the help of computer graphics, motion capture technology, image recognition technology and visualization technologies, Blue Hat accurately “places” virtual objects into the physical world, creating a new and stimulating visual environment for users.
Blue Hat recently displayed a variety of its sci-tech products at the Guangzhou International Toy Exhibition in China including AR Racer, Elastic Bubbles, AR Space Track, AR Alloy Toy Car, AR Need a Spanking, 5D Animated Magic Aquarium, Bug Travelers, AR Picture Book and other interactive games and smart toys.
The company has multiple products in development including new generations of four primary product lines and two new product lines.
Patents and Copyrights
Blue Hat’s advanced AR technology in interactive entertainment is protected by 178 authorized patents with 44 patents in various stages of the application process.
Another 14 applications for Patent Cooperation Treaty, or PCT, have been filed for international patents. As of March 31, 2019, the company owns 645 copyrights for artwork, 71 registered trademarks and 27 software copyrights.
Sales and Marketing
There has been rapid growth in the toys and games industry in China over the last several years. Total retail sales of toys and games in China soared from RMB 111.8 billion in 2012 to RMB 276.5 billion in 2017 with an average annual growth rate of 19.9% in 2017. Blue Hat believes the company is well positioned with little competition as the toy industry rapidly shifts toward intelligent and interactive toys and games. Retail sales of electronic toys grew at 24% annually in 2017 while that of traditional toys grew at 7%.
In addition to a powerful ecommerce presence, Blue Hat has long-term relationships with partnered distributors that place the company’s AR interactive entertainment products into well-known international retail chains and retail outlets. Blue Hat’s integrated online and offline sales channels include e-commerce giants such as Amazon and Alibaba, retail chain stores and the company’s physical experience store located in Xiamen, China. Blue Hat plans to open or franchise approximately 100 additional stores in China by 2021.
Blue Hat’s community-based platform offers users a highly engaged and interactive community with online communication forums and offline social activities. The company advocates a new model of “teaching through lively activities” and combines AR technology with education, integrating its products into situational teaching, roleplaying and man-machine interaction. This novel educational experience helps realize optimal transformation of information, creating a knowledge and enhancing cognition.
Management
Director and CEO Xiaodong (Sean) Chen has over 20 years of experience creating, developing and producing toys and games related products. Chen earned his EMBA from Renmin University of China and has been chairman of the board of directors and general manager of Fujian Blue Hat Interactive Entertainment Technology Ltd. since August 2015.
CFO and Director Caifan, who has over 20 years of financial accounting and taxation experience, earned a degree in finance from Hunan University of Finance and Economics. He has served as director, deputy general manager and financial controller of Fujian Blue Hat Interactive Entertainment Technology Ltd. since August 2015.
Jianyong Cai, chief technology officer and director, has over 35 years of experience in data communication principles, communication network foundation, software engineering, communication network theory and technology and computer network architecture. He holds degrees in data communication principles, communication network foundation and software engineering from University of Science and Technology of China. He has been director, deputy general manager and chief engineer of Fujian Blue Hat Interactive Entertainment Technology Ltd. since January 2010.
BlockQuarry Corp. (OTC: BLQC)
BlockQuarry Corp. (OTC: BLQC), through its in-house initiatives and strategic partnerships, has invested in growing operations targeting the telehealth and cryptocurrency mining industries.
The company specializes in strategic brand development and early growth facilitation. Management maneuvers its proprietary companies through critical stages of market development, including conceptualization, go-to-market strategies, engineering, product integration and distribution efficiency.
Mission
The company’s core mission is to enhance these sectors by implementing innovative services and products that are ready to meet the demands of a changing world. To that end, ISW Holdings leverages its strategic expertise, resources and innovative software to establish market-leading companies and partnerships, thereby ensuring success in their chosen industries.
Cryptocurrency Mining
The start of 2021 saw a massive resurgence in interest surrounding bitcoin and cryptocurrency mining. In mid-February, bitcoin prices hit an all-time high of greater than $57,000, and heightened demand for cryptocurrency mining power has played a key role in exacerbating a global shortage of semiconductors and computer components.
With a foothold in the cryptocurrency mining space, ISW Holdings has placed significant focus on expanding its position and capitalizing on this momentum. Recent highlights include:
- February 9, 2021: The company announced that its revolutionary Pod5 Cryptocurrency Mining Pod will be powered up into full operational launch at the Bit5ive renewable energy cryptocurrency mining facility in Pennsylvania on February 12, 2021.
- February 11, 2021: The company announced that it is in negotiations to purchase a large number of miners (between 300 and 900) in preparation for its coming Phase 3 expansion in mining volume.
- February 23, 2021: The company announced its entry into a comprehensive Hosting and Maintenance Agreement prior to going online with its new ASIC s17 miners.
- March 2, 2021: The company announced that it has successfully tripled its active cryptocurrency mining fleet with the addition of two new POD5IVE datacenters.
“As we continue to bring our miners online, we want our shareholders to be able to track the expansion and profitability of the company’s mining activity given the sharp rising trend in bitcoin prices,” Alonzo Pierce, President and Chairman of ISW Holdings, stated in a news release. “It currently costs about $11K in computing power to mine a single bitcoin. Bitcoin is pricing at over five times that level, making this is an exceptional ROI opportunity, and our responsibility to our shareholders is clear: continue to invest, expand and execute.”
Business Innovations
ISW Holdings’ diverse portfolio reflects the growing demand for essential services in a dynamic modern operational landscape. Some of the company’s current holdings and partnerships include:
- Bit5ive LLC: ISW Holdings operates a joint venture with Bit5ive, a global leader in cryptocurrency mining. The joint-venture agreement enables ISW Holdings to collaborate with the experienced team at Bit5ive to innovate the infrastructure needed to run profitable and efficient crypto mining projects.
- Proceso LLC: ISW Holdings has partnered with Proceso LLC to create high-density processing and mobile data centers powered by renewable energy. These innovations will allow Proceso to offer lower-cost and diverse services to its clients, including hosting and colocation services to growing sectors such as the gaming industry and cryptocurrency mining.
- PHH Health: The company’s home health division answers the growing need for home care services in a world where health care delivery is changing and an increasingly large aging community is looking for efficient and effective ways of accessing health care.
- Volum: The company’s logistics and supply chain management division is designed with the core goal of increasing supply chain efficiency, which is recognized as one of the key aspects of successfully growing any business.
Market Opportunity
ISW Holdings’ recent activity in the cryptocurrency mining sector has positioned it to capitalize on the forecast expansion of the cryptocurrency market in the coming years. According to data from MarketsandMarkets, the cryptocurrency space was valued at $1.03 billion in 2019 and is projected to reach $1.40 billion in 2024, achieving a CAGR of 6.18% during the forecast period.
The report suggests that major drivers for this growth will be the transparency of the underlying blockchain technology, the high volume of remittances in developing countries, the high cost of international remittance, expected fluctuations in monetary regulations and sustained investment in the cryptocurrency space by venture capital firms.
Management Team
Terry Williams is the Chief Executive Officer and Director of ISW Holdings. Mr. Williams brings to the company more than 30 years of experience in accounting and information systems, logistics, insurance and transportation. With a Bachelor’s and Master’s degree in accounting and management information systems, he amassed considerable corporate experience at UPS (NYSE: UPS), where he took several logistical roles, managing more than 2,000 employees and a budget of more than $10 billion. Mr. Williams also serves as president of Airware Transportation and Logistics and Chief Financial Officer of AVI Insurance Caribbean. In 2013, he received the National Airport Minority Advisory Council Award for mastering skills in the aviation industry.
Alonzo Pierce is the company’s President and Chairman. He brings a wealth of business development and wealth management experience to the ISW team, having spent the past 20 years building recognizable brands in multiple industry sectors. Mr. Pierce has launched enterprises in life-styled brands which were delivered to high-profile, high-net worth families and individuals. He has worked in the adult beverage industry, establishing a formidable background in marketing and brand creation. Pierce has a B.A. from Baylor University and has received multiple awards in the adult beverage industry, including ‘Outstanding Sales Performance in the Southern Region’ for Sapphire Brands. Pierce also served as a national liaison to a Super-Regional Bank’s private wealth division. In addition to his for-profit endeavors, Pierce has served on multiple charitable boards, sourcing funding for JRA, food insecure families and housing insecure families.
Kristina Mahoney-Brown is Secretary, Treasurer and Director of ISW Holdings. With more than 20 years of experience providing tax and financial consulting to real estate companies, as well as investors, developers and construction companies, Ms. Mahoney-Brown has gained solid business expertise and market knowledge and prides herself on staying abreast of the latest industry trends. Her professionalism, impeccable work ethic and advanced marketing strategies have earned her the nickname ‘The Tax Diva’. Mahoney-Brown has a Bachelor’s in accounting, a Master’s in taxation and a Master’s in business administration, specializing in personal financial planning.
Brain Scientific Inc. (OTCQB: BRSF)
Brain Scientific Inc. (OTCQB: BRSF) is a commercial-stage health care company focused on developing innovative and proprietary medical devices and software. With a mission of modernizing brain diagnostics by employing cutting edge technologies to bridge the widening gap in access to quality care, the company offers two FDA-cleared products that provide next-generation solutions to the neurology market.
The company’s proprietary, clinical-grade neurological devices are supported by its intellectual property portfolio featuring patents in the United States, China and Europe.
Brain Scientific’s first commercialized devices, NeuroCap(TM) and NeuroEEG(TM), are designed to disrupt the current electroencephalogram (EEG) market by offering cost-effective and disposable substitutes to existing solutions, allowing medical professionals to collect diagnostic information quickly.
The company’s goal is to improve diagnostics by leveraging artificial intelligence and machine learning processes to analyze a database of brain readings as a method of detecting seizures and dementia. The company is also working to improve patients’ access to neurological care.
Headquartered in New York, Brain Scientific and its predecessor (and now wholly owned subsidiary, MemoryMD Inc.) was founded in 2015 and went public in 2018.
Brain Scientific’s first phase of development, from 2018 to 2019, saw the inception of portable, clinical-grade, easy-to-use neurological devices. The second phase, currently ongoing, aims to create cloud-based, secure infrastructure to transmit patient data between patients and their neurologists. The company’s third phase of development is scheduled for 2021-2022 and is expected to focus on the use of AI-assisted diagnostic analysis to increase the efficiency, consistency and accuracy of neurology specialists.
NeuroCap(TM) – Disposable EEG Headset
The NeuroCap is a disposable pre-gelled EEG headset featuring 22 electrodes and 19 active EEG channels, all adhering to the international 10-20 system. The NeuroCap was FDA-cleared in 2018. The headset can be used for recording EEGs in virtually any setting, including urban and rural emergency departments, neurology clinics, urgent care clinics, ICUs, nursing homes, assisted living facilities and remote clinical research labs.
Through a universal cable adapter, the NeuroCap is compatible with other EEG amplifiers. The cap also works in parallel with Brain Scientific’s NeuroEEG amplifier, initiating EEG studies in less than five minutes.
The company is currently seeking FDA approval for additional features for the NeuroCap, as the device has the potential to fill a gap in EEG testing availabilities during the current coronavirus pandemic: in October 2020, Brain Scientific filed an Emergency Use Authorization (EUA) application. The EUA is required for the rapid distribution of the NeuroCap device to emergency departments, intensive care units and other treatment centers to administer prescriptive EEGs safely on critically ill patients or those suspected of being diagnosed with COVID-19.
With more than 80 percent of hospitalized patients infected with COVID-19 displaying neurological symptoms, the NeuroCap could prove to be a valuable device by offering fast testing with limited contact between technicians and patients.
NeuroEEG(TM) – Miniature and Portable Wireless EEG Amplifier
The NeuroEEG is a compact, portable and affordable wireless EEG amplifier intended for prescription use. The 16-channel, FDA-cleared, clinical-grade device acquires, records, transmits and displays electrical brain activity for patients of all ages.
Both the NeuroCap and NeuroEEG are delivered by MemoryMD Inc., a wholly owned subsidiary of Brain Scientific.
Products in Active Development
Currently, Brain Scientific and MemoryMD are working on leveraging their existing products and drawing from ongoing research to develop and commercialize the next generation of solutions for the brain diagnostics market. The devices under development are being designed to address the following issues:
Routine EEG
- NeuroCap-8 is an 8-channel EEG cap. The reduced number of electrodes is vital in emergency room situations, where the time it takes to set up the EEG is critical.
Pediatric EEG
- NeuroCap Pediatric is positioned to become the first disposable and pre-gelled headset available for the pediatric market.
Long-Term Monitoring
- NeuroCap LTM for adult and pediatric patients is a disposable cap designed to monitor rhythmic and periodic patterns for up to 72 hours, providing essential diagnostic capabilities.
- NeuroEEG 24 Channel Amplifier is a portable and wireless amplifier with over 24 hours of battery life.
Artificial Intelligence
- Brain E-Tattoo is a minimally invasive four-channel EEG electrode designed for long-term monitoring.
- An AI database of brain biomarkers collects data on both normal and abnormal brain data to detect neurological diseases. The goal is for machine learning algorithms to enhance understanding of brain-behavior related to epilepsy, memory dementia and pre-Alzheimer’s diagnostics.
Telemedicine
Brain Scientific is expanding the vision for telemedicine in neurology. The company aims to address the current acute neurologist shortfall (20 states have less than 10 neurologists per 10,000 patients) through the use of teleneurology.
Partnership with Marketing Brainology
Brain Scientific has a longstanding partnership with Marketing Brainology, a neuromarketing firm using neuroscience approaches to understand consumer behavior. In 2019, Marketing Brainology conducted a study using NeuroCap and NeuroEEG to determine the most effective Super Bowl commercials.
“Thanks to Brain Scientific’s NeuroCap and NeuroEEG, we are able to better understand the art and science of the human decision-making process,” Michelle Adams, Ph.D, Founder of Marketing Brainology, stated in a news release.
In April 2020, Marketing Brainology again conducted a study leveraging Brain Scientific’s disposable EEG cap to determine how brains were reacting to COVID-19 messaging. Subjects were presented with multiple media impressions, and Marketing Brainology analyzed their responsive biomarkers. The results identified the most effective messaging for engaging with an audience during a crisis.
Market Outlook
The current global market for EEG devices is estimated at $956.1 million. It is expected to rise with a CAGR of 8.7% from 2019 to 2026, reaching $1.6 billion in value by 2026, according to Grandview Research.
In total, there are approximately 6,150 hospitals in the U.S., according to the American Hospital Association. Critically, though, just 254 of those hospitals are certified Level 4 Epilepsy centers with 24/7 EEG coverage. Since very few non-Level 4 centers have extensive EEG tech coverage, this creates a significant opportunity for Brain Scientific to bridge the gap by providing over 5,900 hospitals with lower cost amplifiers and disposable EEG caps.
The company also see opportunities to work with other businesses, such as EEG manufacturers hoping to package Brain Scientific’s solutions with their products, which could greatly expand Brain Scientific’s addressable target market.
Management Team
Dr. Baruch “Boris” Goldstein, Ph.D., is co-founder and Chairman of Brain Scientific. He is a seasoned executive with a proven talent for aligning global business strategies with established and emerging management teams. Goldstein’s growth-focused leadership style has helped him raise over $750 million in venture capital for the development of innovative companies and startups in diverse industries, including financial services, biomedicine, alternate energy and new materials, as well as groundbreaking work in artificial intelligence. His recent achievements include important advancements in neurology and unlocking the potential of AI correlations and machine learning applied to life sciences and medical research. He built a suite of first-to-market companies as a technology-oriented leader, including Ryah Medtech, Brain Scientific, GrapheneCA, E-Forex and Intelligent Video Systems. He also co-founded BrainRX, a company specializing in pre-Alzheimer’s diagnostics.
Dr. Nikolay Kukekov, Ph.D., is a Director of Brain Scientific and a partner at HRA Capital. Before joining HRA Capital, Kukekov was Managing Director of Healthcare Investment Banking at Summer Street Research. His scientific background includes a bachelor’s degree in Molecular, Cellular and Developmental Biology from the University of Colorado at Boulder. He earned his Ph.D. in neuroscience from Columbia University – College of Physicians and Surgeons in New York.
Stuart Bernstein is the company’s Vice President of Marketing. He was recently named to the role after spending the first part of his professional career in senior technical management roles with Fortune 500 companies such as NCR (NYSE: NCR), IBM (NYSE: IBM) and Control Data Corp. He was the CEO of BioSignal, an EEG medical device company. He is also a co-founder of several software engineering and telemedicine firms. One of them, Brain Saving Technology, is now Specialist on Call (SOC Telemed) – a leading telemedicine company that powers over 850 facilities for teleneurology, telepsychiatry and critical care telemedicine with over 200 physicians.
Cannabis Strategic Ventures Inc. (OTC: NUGS)
Cannabis Strategic Ventures Inc. (OTC: NUGS) is an emerging leader in the U.S. cannabis marketplace as a publicly traded cannabis cultivator. The company is based in Los Angeles, with a 6-acre cannabis farm in Northern California called NUGS Farm North. The company’s vision is to acquire and scale assets in the legal cannabis market while achieving efficiencies through economies of scale and vertical integration.
Cannabis Strategic Ventures recently expanded its portfolio by completing the transfer process for cultivation, retail, distribution and manufacturing licenses issued by the City of Los Angeles and the State of California, and it is now working toward taking operational control of each license. The company also recently announced the upcoming grand opening of its cannabis dispensary, MDRN Tree. Following that launch, Cannabis Strategic Ventures intends to deploy another of its new licenses to establish an indoor cultivation facility with capacity to produce two to three pounds of premium exotic cannabis flower per light per harvest. The facility will have up to 1,200 grow lights and is anticipated to yield 5.75 harvests per year, bringing it to a total production capacity of over 15,000 pounds of cannabis flower annually.
Brand Portfolio
The company owns multiple brands under the Cannabis Strategic Ventures umbrella. The firm’s NUGS brand provides operational and financial strategic partnerships and a range of essential services to emerging and existing cannabis consumer brands.
The NUGS Farm North brand operates as a six-and-a-half-acre cannabis cultivation property located in northern California. The company believes that the key to success in its business is consistent quality and reliable supply to fit growing consumer demand. Cannabis Strategic Ventures addressed these consumer needs by building NUGS Farm North. At NUGS Farm North, the company’s process is customized, and its product is consistent. Located in the heart of an agricultural mecca for globally distributed produce, NUGS Farm North finds power in its product, not in its size. Decades of agricultural experience and a dedication to consistency ensure quality cannabis.
MDRN Tree is Cannabis Strategic Ventures’ customer-facing dispensary brand. MDRN Tree will open its first Los Angeles location sometime in the fall of 2021. MDRN Tree will be the company’s factory retail store – a direct interface with the end-market community – where Cannabis Strategic Ventures plans on showcasing the cannabis flower produced at its NUGS Farm North cultivation site. This farm-to-sale model offers the potential to drive simultaneous gains in quality control and profitability.
Market Outlook
The demand for legal marijuana is expected to surge due to ongoing changes in U.S. state government policies toward cannabis. In addition, the number of indications for which medical marijuana is prescribed continues to increase steadily. These factors are expected to rapidly boost legal sales of cannabis products, opening new revenue channels for producers and retailers. Furthermore, an anticipated federal legalization of medical marijuana in the U.S. will only present more high growth opportunities for this market.
According to a report from Grand View Research, the global legal marijuana market was valued at $9.1 billion in 2020. Market size is forecast to grow at a compound annual growth rate of 26.7 percent from 2021 to 2028. That CAGR would put the market value at roughly $30 billion as soon as 2025.
According to the report, “One of the major factors fueling market growth is the expanding demand for legal marijuana owing to the growing number of legal cannabis countries. (Due) to recent legalizations in different countries, the use of medical marijuana for various ailments is gaining momentum worldwide. Patients suffering from chronic illnesses such as Parkinson’s, cancer, Alzheimer’s, and many neurological disorders are administered medical marijuana. The demand for cannabis oil is increasing rapidly, especially among countries with legalized medical marijuana.”
Management Team
Simon Yu is CEO, President, CFO and Secretary of Cannabis Strategic Ventures. He is also a co-founder, former COO and board member of Clubhouse Media Group Inc., a publicly traded social media company. Mr. Yu holds an MBA from the University of Southern California.
Cerberus Cyber Sentinel Corp. (NASDAQ: CISO)
Cerberus Cyber Sentinel Corp. (NASDAQ: CISO) is an industry leader in cybersecurity and compliance services. The company leverages an integrated approach to reduce noise and bridge common silos that often limit the effectiveness of cybersecurity programs. Pulling disparate technologies, teams, and vendors together, Cerberus helps its clients enjoy a simpler and more successful journey to cyber resilience. Since 2019, Cerberus Sentinel has worked to rapidly expand by acquiring world-class cybersecurity and compliance businesses with top-tier talent who utilize the latest technology to create innovative protection solutions.
The Cerberus Sentinel workforce is comprised of cybersecurity experts spanning not only global geographies, but also specialties, industries, regulatory frameworks and focus areas. Its team includes audit and compliance specialists, certified forensics experts, ethical hackers, IEEE® certified biometric professionals, security engineers, around-the-clock analysts, and more – all backed by the most respected credentials in the industry. On an ongoing basis, the company works to identify cyber talent that is culturally aligned and that offers operating leverage through both existing customer revenue and relationships.
Cerberus Sentinel has invested in enterprise solutions and executive talent to integrate its different organizations into an ecosystem that works together to provide complete cybersecurity through cross-pollination of solutions that begin at the network level and extend through technologies, people, policy, and practices. This ecosystem is intended to foster additional growth opportunities and drive overall recurring revenue. Once engaged, the company strives to become trusted advisors for customers’ cybersecurity and compliance demands by providing tailored security solutions based upon their organizational needs.
While cyber resilience requires cycles of continuous improvement, it is a journey that few in the current business and security climate seem to understand. With its deep bench of seasoned experts, Cerberus Sentinel works to simplify that journey for its growing customer base, straightening out the curves and speeding up the process to resilience along the way.
Cybersecurity is a Culture, Not a Product
Integrating compliance and security, including principles of security by design, Cerberus Sentinel helps its clients create an organization-wide culture of cybersecurity. Its offerings include audit and compliance, security operations center services, security engineering, virtual Chief Information Security Officer services, incident response, certified forensics, technical assessments and cybersecurity training.
In contrast to the majority of cybersecurity firms that specialize in a specific technology or service, Cerberus Sentinel seeks to differentiate itself by remaining technology agnostic, focusing on accumulating highly sought-after subject matter experts. Cerberus Sentinel believes that bringing together a world-class team of technological experts with multi-faceted proficiency in the critical aspects of cybersecurity is key to providing technology agnostic solutions to its clients in a business ecosystem that suffers from a chronic lack of highly skilled professionals.
Cerberus Sentinel’s goal is to create a culture of security and to help quantify, define and capture a return on investment from information technology and cybersecurity spending. Its end-to-end, holistic process covers every aspect of clients’ cybersecurity and compliance requirements in an effort to promote greater efficiency and strengthen awareness about the integral role of internal team members in the cybersecurity culture of an organization.
As a result of this strategy, Cerberus Sentinel customers receive an efficient engagement from a single partner that covers a wide range of their needs – addressing challenges more thoroughly and resolving problems more rapidly when compared to working with a host of vendors.
Market Outlook
According to an analysis by the firm Research and Markets, the global managed security services market was valued at $22.45 billion in 2020 and is projected to reach $77.01 billion by 2030, growing at a CAGR of 12.8% through the forecast period.
An expected increase in cybercrime, cost effectiveness of provided solutions and stringent mandatory government regulations aimed at protecting corporate data will drive the global managed security services market for the foreseeable future.
In addition, the documented and growing use of mobile devices in the workplace and the rise in captured and stored digital data serve to fuel market growth. Moreover, growing awareness about the critical nature of data security, the growing importance of e-business and demand for customized services is expected to offer ample opportunities for expansion of the market during the forecast period.
Management Team
David Jemmett is CEO and founder of Cerberus Sentinel. He has more than 35 years of executive management and technology experience with telecommunications, managed services, and cybersecurity consulting services. He previously held positions as CEO of GenResults, a leading provider of security consulting services and technology solutions, and as CTO and founder at ClearData Networks, a HIPAA-compliant HealthDATA cloud hosting platform.
Dave Bennett is COO at Cerberus Sentinel. Since 2015, he has served on the President’s STEM Advisory Board of Grand Canyon University. Before joining Cerberus Sentinel, he served as Chief Product Officer at Experian Health and as Senior Vice President, Product for Gainwell Technologies. He has also held positions as Vice President and Worldwide Head of Build, Healthcare and Life Sciences at DXC Technology, and as EVP, Product and Strategy at Orion Health.
Ashley Devoto is President and Chief Information Security Officer at Cerberus Sentinel. Over the past 17 years, Devoto has worked with the cybersecurity elite to design, build, and operate world-class cybersecurity programs for large, diverse organizations in both government and commercial enterprises. Prior to joining Cerberus, Devoto served as CISO for Booz Allen Hamilton, as business information security officer (BISO) at Bank of America, and as a cyberspace operations officer in the United States Air Force.
Deb Smith is CFO at Cerberus Sentinel. Prior to assuming that position, she was the company’s EVP, Finance and Accounting. She has also served as SVP, Global Accounting at International Cruise and Excursions Inc., and as Chief Accounting Officer for BeyondTrust, an information security software company. She has also held the positions of Corporate Controller at Aspect Software and Assistant Controller at JDA Software.
CNS Pharmaceuticals Inc. (NASDAQ: CNSP)
CNS Pharmaceuticals Inc. (NASDAQ: CNSP) is a clinical stage biotechnology company specializing in the development of novel treatments for primary and metastatic cancers of the brain and central nervous system.
The company was founded in 2017 and is headquartered in Houston, Texas.
Organ Targeted Therapeutics
The company’s lead drug candidate, Berubicin, is proposed for the treatment of glioblastoma multiforme (“GBM”), an aggressive and incurable form of brain cancer. Berubicin also has potential to treat other central nervous system malignancies. Based on limited clinical data, Berubicin appears to be the first anthracycline to cross the blood brain barrier in the adult brain, and it was the subject of a successful Phase 1 study which found the MDT and produced efficacy data as well.
CNS holds a worldwide exclusive license to the Berubicin chemical compound. The company has acquired all requisite data and know-how from Reata Pharmaceuticals Inc. related to a completed Phase I clinical trial of Berubicin in malignant brain tumors. In this trial, 44% of patients experienced a statistically significant improvement in clinical benefit. In 2017, CNS entered into a collaboration and asset purchase agreement with Reata.
CNS intends to explore the potential of Berubicin to treat other diseases, including pancreatic and ovarian cancers and lymphoma. The company is also examining plans to develop combination therapies that include Berubicin.
CNS estimates that more than $25 million in private capital and grants were invested in Berubicin prior to the company’s $9.8 million IPO in November 2019.
CNS intends to submit an IND for Berubicin during the fourth quarter of 2020 and expects to commence a Phase II clinical trial of Berubicin for the treatment of GBM in the U.S. in Q1 2021. A sub-licensee partner was awarded a $6 million EU/Polish National Center for Research and Development grant to undertake a Phase II trial of Berubicin in adults and a first-ever Phase I trial in pediatric GBM patients in Poland in 2021.
The company’s second drug candidate, WP1244, is a novel DNA binding agent licensed from the MD Anderson Cancer Center. In preclinical studies, WP1244 proved to be 500-times more potent than the chemotherapeutic agent, daunorubicin, in inhibiting tumor cell proliferation. The company has entered into a sponsored research agreement with the MD Anderson Cancer Center to further the development of WP1244.
CNS Pharmaceuticals recently engaged U.S.-based Pharmaceutics International Inc. and Italian BSP Pharmaceuticals SpA for the production of the Berubicin drug product. The company has implemented a dual-track manufacturing strategy to mitigate COVID-19-related risks, diversify its supply chain and provide for localized availability of Berubicin. CNS has already completed synthesis of Berubicin’s active pharmaceutical ingredient (API) and has shipped the API to both manufacturers in order to prepare an injectable form of Berubicin for clinical use.
Global Brain Tumor Therapeutics Market
The high recurrence rate of malignant brain tumors is due to reappearance of focal masses, indicating that a sub-population of tumor cells in these cancers may be insensitive to current therapies and may be responsible for reinitiating tumor growth. This necessitates the development of newer drugs in the market that demonstrate greater efficacy in treating such aggressive cancers.
A global increase in neurological disorders has placed increased attention on cancers of the brain over the past decade. Neurological disorders are becoming one of the most prevalent types of disorders, due to longer life expectancy, greater exposure to infection and an increasingly sedentary lifestyle. Because few treatments for primary and metastatic cancers of the brain exist, costs are high and have acted as a restraint for the brain tumor therapeutics market.
Despite progress in surgery, radiotherapy and chemotherapeutic strategies, effective treatments for brain cancer are limited by a lack of specific therapies for the brain and the difficulty in transporting therapeutic compounds across the blood brain barrier. Therefore, there is a significant need for novel and effective therapeutic drugs and strategies that prolong survival and improve quality of life for brain tumor patients.
Several companies are making significant investments into R&D, which is expected to bring more treatment options to the market in the near future. Industry reports consistently project continued growth in the market.
One report estimates that the global brain tumor therapeutics market will reach a valuation of $2.74 billion in 2023, with the market expected to register a CAGR of 11% during the forecast period from 2018 to 2023. Another report projects that the global brain tumor therapeutics market will reach $3.4 billion by 2025, up from $2.25 billion in 2019 (http://nnw.fm/eDUjp).
Management Team
John M. Climaco is the CEO of CNS Pharmaceuticals. For 15 years, Climaco has served in leadership roles for a variety of health care companies. Recently, Climaco served as the Executive Vice President of Perma-Fix Medical S.A, where he managed the development of a novel method to produce Technitium-99. Climaco also served as President and CEO of Axial Biotech Inc., a DNA diagnostics company. In the process of taking Axial from inception to product development to commercialization, Climaco forged strategic partnerships with Medtronic, Johnson & Johnson and Smith & Nephew.
Christopher Downs, CPA, is the company’s Chief Financial Officer. Downs previously served as Interim Chief Financial Officer and Executive Vice President of InfuSystem Holdings Inc. (NYSE: INFU), a supplier of infusion services to oncologists in the United States. Downs holds a Bachelor of Science from the United States Military Academy at West Point, an MBA from Columbia Business School and a Master of Science in Accounting from the University of Houston-Clear Lake.
Dr. Donald Picker is the Chief Scientific Officer of CNS. Picker has over 35 years of drug development experience. Prior to joining CNS, Picker worked at Johnson Matthey, where he was responsible for the development of Carboplatin, one of the world’s leading cancer drugs, which was acquired by Bristol-Myers Squibb with annual sales of over $500 million. In addition, he oversaw the development of Satraplatin and Picoplatin, third-generation platinum drugs currently in late-stage clinical development.
Sandra L. Silberman, M.D., Ph.D., is the Chief Medical Officer of CNS Pharmaceuticals. Silberman is a hematologist/oncologist who earned her B.A., Sc.M. and Ph.D. from the Johns Hopkins University School of Arts and Sciences, School of Public Health and School of Medicine, respectively, and her M.D. from Cornell University Medical College. She then completed both a clinical fellowship in hematology/oncology and a research fellowship in tumor immunology at the Brigham & Women’s Hospital and the Dana Farber Cancer Institute in Boston, Massachusetts. Silberman has played key roles in the development of many drugs, including Gleevec(TM), for which she led the global clinical development at Novartis. Silberman advanced several original, proprietary compounds into Phases I through III during her work with leading biopharmaceutical companies, including Bristol-Myers Squibb, AstraZeneca, Imclone and Roche.
Correlate Infrastructure Partners Inc. (OTCQB: CIPI)
Correlate Infrastructure Partners Inc. (OTCQB: CIPI), formerly Triccar Inc., through its two subsidiaries, Correlate and Solar Site Design, offers a complete suite of proprietary clean energy assessment and fulfilment solutions for the commercial real estate industry. The company believes scaling distributed clean energy solutions is critical in mitigating the effects of climate change. CIPI is at the forefront in creating an industry-leading energy solution and financing platform for the commercial and industrial sector. The company sees tremendous market opportunity in reducing site-specific energy consumption and deploying clean energy generation and energy efficiency solutions at scale.
The opportunity exists to remove friction between today’s legacy finance process and the needed clean-energy upgrades developed within the company’s program technologies. For the U.S. to reach its 2050 carbon goals, 200,000 commercial buildings must be retrofitted every year until that date. That represents approximately a 5-10x increase over the 2022 industry process run rate.
CIPI announced completion of its acquisition of 100% of the equity of Correlate Inc. and Loyal Enterprises LLC dba Solar Site Design on December 28, 2021. The company notes these acquisitions occurred at a key inflection point of its growth. CIPI currently enjoys channel and sales partnerships with Fortune 250 companies and a strong, proven industry network.
The company’s transparent, leading-edge model changes value delivery for both facility owners and proven solution providers seeking scale. CIPI believes its rapid growth is due to industry demand for actionable, cashflow positive energy programs and the underlying carbon reduction mandates taking effect globally.
CIPI has filed with the SEC for a name change to Correlate Infrastructure Partners Inc., which will more closely reflect its new platform and growth focus. The company has been aggressively moving to rebrand, with efforts including a revised website, investor presentation materials and an investor relations awareness campaign. The company’s shares will continue to trade on the OTCQB Venture Market under the current ‘CIPI’ ticker symbol until changes are approved.
Subsidiaries
Correlate, founded in 2015, is a portfolio-scale development and finance platform offering commercial and industrial facilities access to clean electrification solutions focused on locally-sited solar, energy storage, EV infrastructure, and intelligent efficiency measures. Its unique data-driven approach is powered by proprietary analytics, concierge subscription services, and a highly scalable national fulfillment network to help building owners profit from fully funded, turnkey decarbonization and facility health programs. The platform is designed for commercial and industrial real estate owners seeking to significantly improve net operating income while meeting carbon reduction goals. The platform provides energy programs for commercial property portfolios and requires no upfront capital. Client organizations reduce their risk and generate more profits by leveraging Correlate’s unique payment programs to put more cash in the bank. Deploying Correlate’s strategic energy programs and energy management systems allows property-owning organizations to complete big energy changes across their portfolios.
Solar Site Design, founded in 2013, is a U.S. Department of Energy Sunshot Catalyst winner that provides customer acquisition and project development tools for the commercial solar industry. Its commercial marketplace platform connects highly qualified project opportunities to leading solar construction companies nationwide. The Solar Site Design platform gives commercial and industrial property owners access to the best price for a commercial solar system. Commercial solar analysts provide property owners a site assessment and working project proposal. Solar Site Design’s team of solar engineers finalize the design while approved financing providers help clients explore financing options for their projects. Then, approved contractors in Solar Site Design’s Marketplace bid on the projects, ensuring commercial and industrial property owners get the best estimates for their projects. Solar Site Design’s marketplace process promotes transparency and fair pricing. Its team of experts has nearly 20 years of experience in the solar industry. Only reputable, experienced, certified (NABCEP), licensed, bonded and insured contractors are accepted into the Solar Site Design Marketplace.
Market Outlook
CIPI is in a rapidly growing market with a unique offering to address a total market of more than 5.9 million commercial buildings in the United States, according to the U.S. Energy Information Administration. Currently, the company’s wholly owned subsidiaries, Correlate and the Solar Site Design, have an opportunity pipeline of over $100 million in commercial projects with more than $20 million in awarded backlog. According to the Rocky Mountain Institute, portfolio energy optimization is a $290 billion market in the United States driving deep financial savings and energy efficiency across the commercial sector.
Commercial buildings consume more than 35% of the generated electricity in the U.S. and are underperforming in energy efficiency at every level. These buildings waste energy, emit too much carbon, and are too costly for owners and occupants, but retrofits are not happening at the rate or scale needed.
In today’s real estate market, portfolio property owners own most commercial buildings. Yet most building efficiency work is focused on single buildings, thereby missing the distinct needs of this owner class which has very different needs than traditional owner-occupiers. The diverse nature of commercial buildings, combined with technology and performance uncertainty, make simple energy optimization initiatives – which could greatly reduce energy use and improve building value – financially unattractive, resulting in slow adoption rates. CIPI’s financial instruments and software breakdown this issue known as the “split incentive”, unlocking the majority of the addressable market.
Management Team
CIPI has in place a nationally recognized management team that has been active in the energy market since 2005.
Todd Michaels is President and CEO of CIPI and founder of Correlate. He formerly served as Vice President for Innovation at SunEdison and Senior Director Distributed Solar at NRG Energy. He founded Correlate in 2015 and has 16 years of experience in the energy industry. He graduated from Indiana University with a B.S. in Computer Information Systems.
Channing Chen is CFO at CIPI and Correlate Inc. and brings over 16 years of experience in the solar industry as a developer, financier, and business unit leader. He has held executive management roles at Solar Power Partners (acquired by NRG Energy), where he was a founding employee, SunEdison, and NRG Energy (NYSE: NRG). Most recently, Mr. Chen was founder and Managing Partner at Breakaway Energy Partners LLC – a distributed energy financing and market-making platform. To date, Mr. Chen and his teams have raised over $1.5 billion in financing across residential, commercial, and utility scale solar and energy storage projects representing over 400 MWs. He holds a B.A. in Environmental Chemistry from the University of California at San Diego and an MBA from the University of Southern California. He is also an advisor and early-stage investor to several startup companies in the renewable energy space.
David Bailey is Chief Revenue Officer of Correlate Inc. With over 15 years of executive sales, supply chain management, and energy efficiency experience, he is responsible for ensuring the success of the National Commercial Sales Unit across multiple regional project teams. Mr. Bailey created and launched the Transformation Services team while at Wesco for its multibillion-dollar Distributed Energy Resource division, formerly Westinghouse. His focus was on IoT-enabled efficiency and plant floor automation-based services. Before that, he spent several years in Global Account Sales Management, with GE Supply as a Program Manager, and is a Commercial Leadership Program graduate. Mr. Bailey received his B.S. in Mechanical Engineering from the University of Kentucky.
Jason Loyet is VP of Commercial Sales of Correlate Inc. He is a cleantech executive with over 20 years of experience leading high growth solar energy and software start-ups. Mr. Loyet is a U.S. Department of Energy SunShot Catalyst award winner for his work building the Solar Site Design technology platform. Before joining the solar energy industry in 2005, he founded and sold two software companies in the streaming media (GlobalStreams) and newspaper publishing (MyCapture) industries. Mr. Loyet currently serves as a Member of the Board of Directors for the Tennessee Solar Energy Industry Association (TenneSEIA).
Deke Welling is Head of Project Development and Fulfillment Services at Correlate Inc. He has over 19 years’ experience in the energy industry with an emphasis on renewables and energy efficiency over the past seven years. Prior to entering the renewables sector, Mr. Welling was the CEO of Welling Resources, an energy development company focused on the exploration of oil and natural gas reserves in the U.S. It was this experience that led him into the renewables sector and leading a charge for more sustainable resources. Additionally, Mr. Welling also served as the CEO of Circle L Solar Inc., a top 100 solar installer in the United States since 2016. Through his leadership, Circle L Solar experienced a growth rate of over 2,250% from 2016 to 2019, resulting in his company being listed on the Inc. 5000 list of the fastest growing private companies in the U.S. (Rank #176) and being named ‘Top Energy Company’ and ‘Entrepreneur of Year for the Energy Industry’ by the American Business Awards® in 2019 and again for ‘Entrepreneur of the Year’ in 2021.
Kevin Warren is Head of Construction and Development Engineering at Correlate Inc. He is a solar veteran with over 12 years of experience in the field. Prior to co-founding CLS, Mr. Warren was the owner of Beacon Consulting and has originated, consulted, designed and/or engineered over 122 MW of PV installations ranging from small commercial to utility scale projects throughout Texas, California, Colorado and North Carolina. He holds a Photovoltaic Technical Sales Professional Certification from the North American Board of Certified Energy Practitioners and certifications from Solar Energy International in PV Installation, PV Technical Sales, PV battery-based design, PV design and engineering, and PV operations and maintenance. Along with PV expertise, Mr. Warren is a LEED Green Building Associate, a certified building analyst from the Building Performance Institute, a Certified Renewable Energy Professional from the Association of Energy Engineers and holds a designation in High-Performance Sustainable Buildings from the BOMI Institute. He studied Electrical Engineering at the University of Texas at Arlington.
Tom Kunhardt is Director of Customer Success at Correlate. He previously held a similar position at Clean.Tech and was Corporate Trainer, Learning & Development, at NRG Energy. He has 15 years of experience in the solar and clean energy industries helping homeowners and businesses find solutions to their energy needs. He holds a bachelor’s degree from the University of Massachusetts.
Cub Crafters Inc.

Cub Crafters Inc. (typically styled CubCrafters) is an OEM aircraft manufacturer based at McAllister Field Airport in Yakima, Washington. The company was founded in 1980 to build parts and supplementary type certificate (STC) improvement modifications, which were used to establish it as the preeminent center for rebuilding the classic Piper PA-18 Super Cub light aircraft. CubCrafters went on to advance the market with its own, newly manufactured aircraft models and holds an approved Federal Aviation Administration (FAA) Production Certificate. Yakima-based operations include an engineering design-test-certification center, aircraft parts and assembly production facilities, and an MRO maintenance service and overhaul facility.
The first newly manufactured aircraft by the company, the CC18-180 Top Cub, was Federal Aviation Administration (FAA) type certified in December 2004. The Top Cub was also granted type certificates (TC) by Transport Canada in July 2008, followed by Australian certification in August of that same year. With the FAA’s release of the new Light Sport Aircraft (LSA) class, CubCrafters created a brand-new model in 2008, the CC11-100 Sport Cub, similarly based on the original Piper J-3 Cub’s appearance, which it validated to ASTM international standards as an LSA. This model advanced to become known as the Carbon Cub, the bestselling LSA of all time in the U.S.
CubCrafters focuses on four main product lines, including the Carbon Cub SS, Carbon Cub FX, XCub, and the Top Cub under license. Some models are built to be lightweight and powerful for quicker flights, while others are built for longer missions in unforgiving backcountry environments.
CubCrafters has a service and overhaul facility for PA-18 Super Cubs and other Cub derivative designs at its Yakima headquarters. The company sells aircraft kits as well as finished aircraft.
Aircraft
The Carbon Cub is available in three variants: Carbon Cub SS (production Light Sport Aircraft), Carbon Cub FX (an innovative Builder Assist E/A-B aircraft) and Carbon Cub EX (E/A-B aircraft kit). Carbon Cub has been designed for off-airport operation with a powerful engine, strong lightweight airframe and nimble low-speed manners. The Carbon Cub has taken the fundamentally superior design of the Piper Super Cub and reinvented it using 21st century materials and computer-aided design. Superior engineering results include the Carbon Cub having 50% fewer parts and weighing more than 300 pounds less than a similarly equipped Super Cub. Now in its third generation of innovation advancements, there are over 1,000 Carbon Cubs flying.
The CubCrafters CC19-180 XCub, FAA Certified and introduced in June 2016, is supplied complete and ready-to-fly. The XCub is a further scaled development of the CubCrafters Carbon Cub, which the company continues to supply, but with higher performance and incorporating more structural carbon fiber. The XCub was developed over a six-year period and not publicly announced until FAA TC had been completed and issued. The process was completed organically using company resources and did not involve any venture capital, loans nor any advanced customer deposits. XCub is built on a wholly original fuselage design. The CNC-milled 4130 chromoly steel frame meets the latest FAA Part 23 certification standards for 2,300-pound gross weight aircraft. XCub’s useful load is as high as 1,084 pounds. Current Part 23 certification requirements ensure this is the strongest Cub ever produced. It can fly farther, providing greater comfort. It is an airplane that has taken the best from the past and, using the very latest in design, material and manufacturing technology, has established a new standard.
The XCub was approved by the FAA for seaplane operations in December 2017. That same month, EASA approved the XCub design and issued a new type certificate. Four international type certificates have been gained: EASA Dec-2017, Canada Feb-2018, Japan April-2018, and Australia Aug-2018.
CubCrafters increased the horsepower of the XCub line in 2019, offering two new models: the CC19-215 FAA Certified version and the CCX-2300 Builder Assist, both powered by the new CC393i 215 HP engine built by Lycoming.
In December 2021, CubCrafters gained FAA Certification of a new nose wheel version of the XCub, branded the NXCub.
Market Overview
According to a 2022 analysis by research firm Expert Market Research (“EMR”), the global ultralight and light aircraft market was valued at $7.63 billion in 2021. The EMR report says the market is expected to grow at a CAGR of 4.5% in the forecast period of 2022-2027 to reach a value of $9.93 billion. Ultralight and light aircraft are small aircraft with on-board pilot (and perhaps passengers) designed for use in recreation, sports, pilot training, aerial surveys, mapping, research and agriculture, humanitarian backcountry access, and special military missions, as well as business and personal travel.
CubCrafters currently enjoys a dominant market share of the rugged adventure airplane market.
Management Team
Patrick Horgan is President and CEO at CubCrafters. Before he assumed that role, he was the company’s Vice President/Director of Engineering & Product Development for three years, when he led the FAA Part 23 type certificate approval and production certificate approval of CubCrafters’ newest flagship, the XCub. Mr. Horgan also directed the breakthrough certification that authorized the use of experimental avionics in FAA-certified production aircraft, a first in aviation history. He brings over 30 years’ aircraft development and manufacturing experience in general aviation, commercial, and military industries. Prior to service at the company, he was the General Manager at WACO Classic Aircraft Corporation in Battle Creek, Michigan, and was the commercial aircraft manager of the Boeing 777 wheel and brake program for Goodrich Aerospace in Troy, Ohio. He was also a designer on the F/A-18 Super Hornet at McDonnell Douglas (now Boeing) in St. Louis, Missouri. Mr. Horgan holds degrees in aeronautical and astronautical engineering from the University of Illinois, and a certificate in Disruptive Strategy from Harvard Business School. He serves as a member of the Board of Directors of the General Aviation Manufacturers Association and on ASTM aircraft standards committees.
Brad Damm is Vice President at CubCrafters. He has overseen CubCrafters’ sales, marketing, and brand management operations since 2018. Since first joining CubCrafters in 2013, Mr. Damm has served as Factory Direct Sales Manager, the Director of Sales Support, the Global Director of Sales, and the Vice President of Sales and Marketing. During his tenure, the company has seen new sales records year after year across all of CubCrafters new aircraft and kit product lines, and the CubCrafters brand has risen to new levels of awareness and respect with aviation consumers worldwide. Prior to joining the company, he served for over 10 years as the Business Development Manager for one of the largest commercial concrete contractors in the Pacific Northwest, driving the sales and revenue growth that allowed the company to expand from a few dozen to hundreds of employees.
Rick Johnson is the Director of Finance at CubCrafters and has been with the company since 2017. He has 27 years of previous experience as controller and CFO for fruit packing and timber operations in the Pacific Northwest. He holds a Bachelor of Science in Business Administration from Central Washington University.
Christopher Matus is Production Manager at CubCrafters and joined the company in 2011. Before taking that post, he held positions as Fabrication Plant Manager, Machine Shop Manager and CNC Machinist for the company. He has also served as a Combat Engineer in the Washington Army National Guard, deploying to Afghanistan and to natural disasters including the 2014 Oso Mudslide.
Justin Jansky is the Administrative Manager at CubCrafters. He joined the company in 2015 and has a demonstrated history of successful collaboration on major FAA type certification projects in the general aviation industry, specifically under 14 CFR Parts 21 and 23. He is responsible for process management, document control, facilitating FAA certification processes, coordination with FAA delegates and documenting compliance testing. He holds a bachelor’s degree in technology and applied design.
Cybin Inc. (NEO: CYBN)
Cybin Inc. (NEO: CYBN) (NYSE American: CYBN) is a Canada-based life sciences company focused on the pharmaceutical development of psychedelic products, as well as the functional mushroom market.
The early-stage company boasts an experienced management team featuring industry veterans from pharmaceutical and consumer product backgrounds who have run multiple clinical trials and collectively helped facilitate billions of dollars in product revenues. The team is dedicated to the development of products and protocols within the psychedelic, pharmaceutical and nutraceutical industries.
In particular, Cybin aims to further build upon and expand its intellectual property (IP) portfolio, which is structured around unique psilocybin delivery mechanisms that target a number of different therapeutic indications. In addition, the company has dedicated itself toward furthering its research and IP within the fields of synthetic compounds, extraction methods, the isolation of chemical compounds, new drug formulations and protocol regimes.
Serenity Life Sciences & Natures Journey Inc.
The company’s business model is centered around its two core subsidiaries, Serenity Life Sciences and Natures Journey Inc., which comprise Cybin’s two-pronged approach toward delivering fungi-derived psychedelic and medicinal products.
Serenity Life Sciences is focused on furthering research and development of psilocybin-based medications. Psilocybin is found in certain species of mushrooms and is a non-habit forming, naturally occurring psychedelic compound. Research into psilocybin has shown positive results for the treatment of depression, anxiety, PTSD, addiction, eating disorders, ADHD and other indications.
Natures Journey Inc. operates the Journey brand, which specializes in developing proprietary medicinal mushroom products that target and promote mental wellness, immune boosting detoxification and overall general health and wellbeing.
Partnership with the Toronto Centre for Psychedelic Science (TCPS)
Staying true to its axiom of being a research-first medicinal mushroom life sciences company, Cybin recently announced its entry into a strategic partnership with the Toronto Centre for Psychedelic Science (TCPS), with the goal of furthering its ongoing psilocybin research efforts and expanding Cybin’s psilocybin IP portfolio (http://nnw.fm/9EUkI).
“While there is evidence to support psilocybin as a treatment for certain indications, the Toronto Centre for Psychedelic Science is taking a clinical approach to prove or disprove the safety and efficacy of psilocybin-based microdosing through an open science approach,” Paul Glavine, CEO of Cybin, stated in a news release.
“We are excited to join forces with Cybin and to offer our expertise. A number of firms had approached TCPS, but Cybin demonstrated a superior commitment to high-quality research and integrity in product development. Our high standards for scientific rigor and transparency will find a fitting home within the culture Cybin is cultivating in Canada and abroad,” Thomas Anderson, co-founder of the Toronto Centre for Psychedelic Science, added.
Journey’s Product Monetization & Market Potential for Nutraceutical Supplements
Although Cybin is at the forefront of companies seeking to conduct clinical trials aimed at gaining regulatory approval for psilocybin and other psychedelic products, the company has also placed a great deal of emphasis on generating meaningful revenue from its very outset.
Cybin’s Journey brand has is launching a range of supplements comprised of popular fungi-derived ingredients such as Reishi, Lion’s Mane and Cordyceps. Purported to aid focus and concentration while promoting neurogenesis, Journey’s range of nutraceutical products provides Cybin with a crucial foothold within the non-psychedelic legal supplement market, which is valued at over $25 billion globally and growing at a 9% year-over-year rate.
Pharmaceutical Psychedelics
In addition to the company’s range of non-psychedelic supplements, Cybin has plans to carry out a clinical trial with a new delivery system for its psilocybin-based medications later this year. Ultimately, the company aims to enter into technology transfer agreements with global pharmaceutical companies after phase 1 & phase 2 clinical trials are complete in order to accelerate regulatory approvals in major indications in global markets with entire lifecycle product management.
With products such as psilocybin truffles already legal in nations such as the Netherlands, Jamaica and Bulgaria, Cybin has positioned itself to capitalize on an eventual legalization of psychedelic mushroom-derived products in the future. Working within a regulatory environment with strong similarities to that which dealt with cannabis prior to the industry’s eventual legalization by the Canadian government in 2018, Cybin is laying the groundwork for the moment pharmaceutical psychedelics gain acceptance in North America and abroad.
Amalgamation Agreement and Financing
Cybin recently announced its entry into an amalgamation agreement dated June 26, 2020, with Clarmin Explorations Inc. (TSX.V: CX) and 2762898 Ontario Inc., a wholly owned subsidiary of Clarmin (http://nnw.fm/w04LH). Completion of the transactions contemplated in the amalgamation agreement will result in the reverse takeover of Clarmin by Cybin.
In connection with the proposed transaction, Cybin plans to complete a “best-efforts” brokered private placement of subscription receipts of Cybin, with a syndicate of agents co-led by Stifel Nicolaus Canada Inc. (Stifel GMP) and Eight Capital, to raise a minimum of C$14 million ($10 million) and a maximum of C$21 million ($15 million), with a 15% agents’ option.
To date, Cybin has raised approximately C$10,400,000 through an initial financing round and its series A financing round.
DarioHealth Corp. (NASDAQ: DRIO)
New York and Israel-based DarioHealth Corp. (NASDAQ: DRIO) leads global digital therapeutics (DTx) with its popular, smartphone-centered personalized chronic illness management software-as-a-service (SaaS). The company’s strategic advantages include:
- AI-powered digital solutions that drive durable behavior change in chronic disease patients, and
- Personalized user experience at scale to make behavior change the path of least resistance.
Approximately $3 trillion in annual U.S. costs associated with chronic illnesses like diabetes, hypertension and obesity are largely preventable with behavioral therapies. Formerly limited to periodic office visits, these therapies can now scale to millions with tech-enabled, continual and remote health monitoring, as well as AI-driven digital and live coaching. This is all possible while still maintaining the personalization required for success in reducing illness and its related effects and costs.
Roughly 51,000 active, paying users manage their health with Dario’s platform that combines smartphone-connected vitals measurement, remote patient monitoring (RPM), lifestyle management tools, and AI-driven and human coaching to deliver improved clinical outcomes.
Among the most downloaded medical apps, the Dario platform is rated at 4.9 stars on the Apple App Store and features 11,000 reviews, along with a Net Promoter Score (a measurement of consumers’ willingness to recommend the product to others) that’s the highest in its field.
Company Strategy
Clinical studies demonstrate Dario’s direct improvement on users’ health measures like H1AC scores (diabetes) and blood pressure (hypertension).
Patient engagement in therapies leads to health success. Dario’s platform centers on continual maximization of patient engagement through personalization, including ‘nudges’ and live, AI-generated responses to health measures provided by Dario’s smartphone-connected medical devices.
Proprietary data analysis provides valuable insights that not only improve health care providers’ medical capabilities but, through artificial intelligence, encourage patients to take evidence-based and highly personalized preventative measures that reduce risk, emergency room visits and preventable hospitalization.
Dario is now deploying its successful B2C platform in B2B2C, targeting employers and health plans with competitive advantages in cost, software and hardware.
The company estimates an annual addressable U.S. market of $72 billion, only 1% of which has been penetrated with digital therapeutics.
The strategic transition to B2B2C (from exclusively B2B) is intended to accelerate revenue growth by reducing Dario’s cost per acquisition per user and expanding margins.
Dario’s commitment to aggressive growth is also shown by its appointment of a new president, chief medical officer and head of sales for North America, all from a highflyer behavioral health company.
Key growth drivers planned include expansion of the company’s paying B2C subscriber base; lateral expansion into other chronic conditions that overlap with its core diabetes populations, such as hypertension, obesity and depression; and increased B2B2C penetration.
Financial Highlights
The company plans to leverage a massive opportunity for growth, with a global addressable market for digital therapeutics of roughly $108 billion. In the U.S. alone, that number is estimated at $72 billion, and only about 1% of that market has been penetrated.
Dario’s strategic transition to an SaaS membership business model increased gross profit by 87% in Q1 2020, as compared to the prior year. Membership revenue increased from 27.1% to 46.7% in the same period. The company is seeing improved operating efficiencies as it shifts focus to the B2B2C business model, and it expects average revenue per user per month (ARPU), which was $6 and $25 in 2019 and 2020, respectively, to reach $70.
Value to Consumers and Businesses
Dario continually evaluates and optimizes the value and return its platform delivers to consumers and businesses.
Consumers seeking to understand how their everyday behavior impacts their personal health and chronic conditions benefit from actionable feedback on how to improve health and better collaborate with health care providers.
Businesses looking to increase employee satisfaction, loyalty and productivity with fewer health-related absences take advantage of Dario’s services for employers.
Health care providers improve patient compliance using the platform’s interactive services that allow for greater monitoring, which improve engagement with patients at the right times and with the right treatments.
Health plans can leverage DarioHealth’s solutions to improve patient outcomes and lower costs.
Recent Studies
The company recently presented the results of two new studies at the American Diabetes Association’s 80th Scientific Sessions, which showed sustained improvements in blood glucose levels and blood pressure among users of its digital therapeutic platform for chronic diseases. The results of these two studies demonstrate that the use of Dario’s therapeutic platform promotes behavioral modification, enhanced individual engagement and improved clinical outcomes.
Remote Patient Monitoring (RPM) Agreements
The Centers for Medicare & Medicaid Services recently approved RPM codes for Medicare patients, which enables physicians to bill for between-visit patient care.
This simplifies implementation of the company’s open and scalable AI-driven platform and further supports transition to the company’s high-margin, recurring SaaS model targeting B2B2C revenue channels.
Emergency COVID-19 FDA Guidelines Allow Self-Test Blood Glucose Meters
In an effort to preserve personal protective equipment (PPE) and reduce contact between health care providers and patients in hospital settings due to COVID-19, the U.S. Food and Drug Administration (FDA) has recognized that home-use blood glucose meters, including Dario’s smartphone-connected metering device, may be used by patients with diabetes who are hospitalized due to COVID-19 to check their own blood glucose levels and provide the readings to the health care personnel caring for them.
As a result, hospitals can now allow patients to self-test using their Dario blood glucose testing strips and smartphone-connected devices, or hospitals can issue patients Dario devices upon admission for COVID-19-related conditions.
Irregularities in blood glucose levels are suspected as a factor in the increased severity of potentially deadly COVID-19 complications. As such, a high priority is being placed on stabilization of patients’ blood glucose levels.
Awards and Recognition
DarioHealth’s Blood Glucose Monitoring System was voted as the ‘Best Glucometer for Data Management’ by Top Ten Reviews. Jeph Preece, senior editor at Top Ten Reviews, said, “The Dario app is the best data management system that I’ve seen. Compared to apps by popular brands, Dario’s system looks and feels like it’s years ahead of the curve.”
‘The Global Digital Health 100’, an annual award sponsored by the reputable Journal of Health, recognized DarioHealth as a leader among health technology companies demonstrating the greatest potential to change the way that health care is delivered.
D-Wave Quantum Inc. (NYSE: QBTS)
D-Wave Quantum Inc. (NYSE: QBTS) is a leader in quantum computing systems, software and services focused on delivering customer value via practical quantum applications for problems such as logistics, artificial intelligence, materials sciences, drug discovery, scheduling, fault detection and financial modeling. As the only provider building both annealing and gate-model quantum computers, the company is unlocking commercial use cases in optimization today, while building the technologies that will enable new solutions tomorrow.
D-Wave is a pioneer in quantum computing, with a history of delivering the world’s first commercial quantum computer; the first real-time quantum cloud service; countless hardware and software product and research milestones; and the planned first cross-platform quantum solution which will deliver both annealing and gate-model quantum computers to customers via an integrated platform. Its current commercial product offerings include: Advantage™ (fifth generation quantum computer), Leap™ (quantum cloud service), Launch™ (quantum computing onboarding service) and Ocean™ (full suite of open-source programming tools).
D-Wave’s relentless pursuit of practical quantum computing has resulted in the technology being used today by some of the world’s most advanced enterprises – more than 25 of the Forbes Global 2000 use D-Wave.
D-Wave’s commercial customers include blue-chip industry leaders like Volkswagen, Accenture, BBVA, NEC Corporation, Save-On-Foods, DENSO and Lockheed Martin. The company boasts an extensive IP portfolio featuring more than 200 issued U.S. patents and over 100 peer-reviewed papers published in leading scientific journals.
Founded in 1999, D-Wave is the world’s first commercial supplier of quantum computers. With headquarters and the Quantum Engineering Center of Excellence based near Vancouver, Canada, D-Wave’s U.S. operations are based in Palo Alto, California.
Advantage™ Quantum Computer
With the Advantage™ Quantum Computer, D-Wave has incorporated two decades of experience and over 10 years of customer feedback to create the first and only quantum computer designed for business. The platform features a new processor architecture with over 5,000 qubits and 15-way qubit connectivity. This is 2.5x more connections and more than double the number of qubits than the company’s previous generation quantum computer.
D-Wave’s quantum computers, first located in its facilities in British Columbia, have been available to North American users through its Leap™ quantum cloud service since 2018. It has since introduced new Advantage systems in Julich, Germany, and most recently, Marina Del Rey, California, which marked the availability of the first Advantage quantum computer physically located in the United States.
That new deployment is part of the USC-Lockheed Martin Quantum Computing Center (QCC) hosted at USC’s Information Sciences Institute (ISI), a unit of the University of Southern California’s prestigious Viterbi School of Engineering. Additionally, Amazon Web Services (AWS) and D-Wave announced that the U.S.-based system is available for use in Amazon 2racket, expanding the number to three different D-Wave quantum systems available to AWS users.
Leap Quantum Cloud Service
D-Wave’s customers interface with its systems through the Leap™ quantum cloud service. Leap delivers immediate, real-time access to the company’s Advantage quantum computer and quantum hybrid solver service, all with enterprise-class performance and scalability.
Leap allows developers proficient in Python to get started building and running quantum applications. Through a seamless and secure cloud-based connection, users can easily start solving complex problems of up to 1 million variables and 100,000 constraints.
Using Leap, D-Wave customers have developed quantum hybrid applications for use cases in manufacturing, logistics, financial services, life sciences, materials science, retail and transportation. By eliminating the need to wait hours, days or weeks to get good answers to a broad array of problems, D-Wave is helping businesses move forward.
D-Wave Launch
D-Wave Launch™ is the company’s onboarding platform aimed at helping businesses easily start their quantum journey. Through this program, D-Wave’s team of experts and partners aid enterprises in identifying best use cases for quantum and work with them to develop a proof of concept and production pilot.
From there, the team coordinates with customers to get their hybrid quantum applications up and running, providing ongoing Leap quantum cloud access to ensure the application is operating smoothly and delivering real business value.
Target Verticals
While the potential applications for quantum computing are effectively limitless, D-Wave has identified a number of industry verticals as key areas of focus for its quantum architecture, providing case studies for each. These include:
- Manufacturing – D-Wave worked with Volkswagen to identify a commercial optimization application, the binary paint shop problem, which was run on D-Wave’s hybrid solver service. The solver outperformed four purely classical methods on problem sizes at commercial scale (N=3,000). In a separate project, similar inputs were tested using a leading ion trap system, which failed to find any commercial solution.
- Life Sciences – Menten AI makes use of D-Wave quantum computing to assist in the design of novel therapeutic peptides—short strings of amino acids that can act as potent drugs. With the rise of COVID-19, D-Wave’s Advantage system made it possible to identify molecules that might be especially well-suited for binding and inhibiting the related spike protein, producing several promising peptide designs.
- Finance – Multiverse Computing, a leader in developing quantum solutions for the financial sector, leveraged D-Wave’s hybrid solver service in a collaboration with BBVA, one of the world’s largest financial institutions. Multiverse demonstrated management strategies that far exceeded the granularity of traditional returns in a fraction of the time, helping BBVA identify a low-risk portfolio for investment.
Market Opportunity
The quantum computing total addressable market is projected to grow between $450 billion and $850 billion over the next 15 to 30 years, with between $5 billion and $10 billion of anticipated TAM growth coming in the next three to five years, according to Boston Consulting Group. Driving factors behind this growth include rising investments in quantum computing tech by governments and an increasing number of commercial use-cases.
Forward-thinking organizations see quantum as an opportunity to move ahead of the competition. From finding efficiencies and reducing waste to decreasing time to solution and solving problems abandoned due to complexity, the business value is real. According to data from 451 Research, 40% of large enterprises are already experimenting with quantum computing.
D-Wave is strategically positioned – in an industry with significant barriers to entry – as evident by a decades-long track record serving a roster of blue-chip customers. The company is singularly focused on helping its customers achieve clear value by leveraging quantum computing in practical business applications. With a full stack of systems, software, developer tools and services, D-Wave is working to enable enterprises, governments, developers and researchers to access the power of quantum computing, thereby providing an intriguing opportunity for prospective investors.
D-Wave’s current investor base includes PSP Investments, Goldman Sachs, BDC Capital, NEC Corporation, Aegis Group Partners and In-Q-Tel.
Leadership Team
Dr. Alan Baratz has served as the CEO of D-Wave since 2020. Previously, as Executive Vice President of R&D and Chief Product Officer, he drove the development, delivery, and support of all of D-Wave’s products, technologies, and applications. Dr. Baratz has over 25 years of experience in product development and bringing new products to market at leading technology companies and software startups. As the first president of JavaSoft at Sun Microsystems, he oversaw the growth and adoption of the Java platform from its infancy to a robust platform supporting mission-critical applications in nearly 80 percent of Fortune 1000 companies. He has also held executive positions at Symphony, Avaya, Cisco, and IBM. Dr. Baratz holds a doctorate in computer science from the Massachusetts Institute of Technology.
John Markovich is the company’s CFO. He brings to D-Wave over three decades of experience working with rapidly growing private and public technology companies across all stages of development. Mr. Markovich has directed the finance, accounting, tax, treasury, M&A, legal, operations, customer service, IR, HR, and IT functions for companies ranging from privately held pre-revenue startups to an NYSE-listed Fortune 500 multi-national company with over $1.2 billion in annual revenue. During his career, he has negotiated and closed over 150 debt, equity, M&A, and joint venture transactions exceeding $2.5 billion in value; over a dozen private placements; nearly a dozen M&A transactions; and several international joint ventures. Mr. Markovich holds a BS in Business from Miami University and an MBA from the Michigan State Graduate School of Business.
Delic Holdings Corp. (CSE: DELC) (OTCQB: DELCF)
Delic Holdings Corp. (CSE: DELC) (OTCQB: DELCF) is the leading psychedelic wellness platform, committed to bringing science-backed benefits to all and reframing the psychedelic conversation. The company owns and operates an umbrella of related businesses, including trusted media and e-commerce platforms like Reality Sandwich and Delic Radio; Delic Labs, the only licensed entity by Health Canada to exclusively focus on research and development of psilocybin vaporization technology; Meet Delic, the premiere psychedelic wellness event; and Ketamine Infusion Centers, one of the largest ketamine clinics in the country.
Delic is backed by a team of industry and cannabis veterans and a diverse network, whose mission is to provide education, research, high-quality products, and treatment options to the masses. Its founders helped build the multi-billion-dollar cannabis industry and aim to do the same in psychedelics as it follows a similar path toward legalization. In its quest to advance the new psychedelic renaissance upon us, Delic has become the pioneer in its field, creating an ecosystem of opportunities by investing in cutting-edge ideas.
The Vancouver-based company was formed in 2019 to address the growing interest in psychedelic wellness backed by science. Delic was the first psychedelic umbrella platform. It is currently a trusted source for those interested in psychedelic culture, education, treatments, and more.
While other emerging companies focus on patent medicine and big pharma for substances limited by government regulation, Delic is blazing a unique trail. It identifies ancillary and fully legal opportunities like IP, new media, live events, ketamine clinics (with the ability to offer additional psychedelic treatments once legalized, and large-scale production and brings them under its big tent of resources and reach.
The Big Problems Delic Is Addressing
- Fifty percent of Americans will meet the criteria for a mental health condition sometime in their lifetime. The FDA has approved psilocybin therapy as a breakthrough therapy for depression.
- Every 40 seconds, someone in the world commits suicide. Ketamine has been shown to decrease thoughts of suicide significantly. In 2019, the FDA approved esketamine as a fast-acting antidepressant.
- Traditional palliative care methods do not eradicate end-of-life (EOL) anxiety. LSD and psilocybin have been shown to reduce EOL anxiety for terminally ill patients. Eighty percent of terminally ill patients with psilocybin sessions experienced significant reductions in depression and anxiety.
- Approximately 50 million people in the U.S. are addicted to some tobacco product. Research shows that psilocybin is helping people quit smoking.
The Delic Ecosystem
The Delic Ecosystem covers three main areas: media, health, and science. The media focus is educating and motivating the masses through a variety of digital platforms, like Delic’s Reality Sandwich digital magazine, a free public education platform providing psychedelic guides, news and culture (1.4+ million page views in 2020 and 54k social media followers across all platforms); Meet Delic, the first-ever psychedelic wellness summit and the premier psychedelic wellness event based in Las Vegas (over 2,000 live attendees and 5,000+ email subscribers); and Delic Radio (over 43 episodes and 100k total streams). Delic has also been featured in numerous media outlets like Forbes, NBC News, The Joe Rogan Experience, Daily Beast, High Times, and The Dr. Drew Podcast.
The focus of Delic’s health operations is the most accessible psychedelic treatments that can help billions of people live happier lives. Delic does this through one of the largest ketamine clinic chains in the country, Ketamine Infusion Centers (KICs), a limited liability corporation formed under the laws of Arizona that runs three ketamine clinics located in Bakersfield, California, and Phoenix, Arizona. Its management team has over 15 years of experience in the clinic and medical space, scaling and operating over 20 clinics, with a plan to open 10 more clinics in the next 18 months. Together, these clinics have overseen 4,000+ treatments delivered to date.
The focus of Delic’s science operations is developing IP and advanced extraction and testing facilities that are the backbone of the legal market. Delic carries this out through Delic Labs, a licensed cannabis and psilocybin research laboratory based in Vancouver. It’s the only entity licensed by Health Canada to exclusively focus on research and development of psilocybin vaporization technology.
Founded by award-winning chemists, Delic Labs focuses on extraction optimization, analytical testing, and chemical process development to advance the cannabis and psilocybin industries. Health Canada gave it a Section 56 Exemption to work with psilocybin compounds, allowing the company to possess and research these products for development and quality control before they hit the market.
Latest Acquisition – Homestead Book Company
On March 4, 2021, Delic announced its acquisition of Seattle-based Homestead Book Company. Homestead is a legacy counterculture distributor of psychedelic media. It’s also the creator of one of the first self-contained psilocybin mushroom grow kits.
The acquisition of Homestead is an exciting one, as it shows how Delic is increasing accessibility to this nascent industry within regulated jurisdictions. Homestead has sold tens of thousands of mushroom kits globally and was one of the earliest distributors for High Times and many other counterculture publications.
The Homestead acquisition allows Delic to increase its product offerings on its website, Reality Sandwich, which recently hit a record for average monthly traffic of over 200,000 unique visitors and over 2.6 million active readers in 2020.
Market Outlook
The psychedelic renaissance is here. Just in time to help address the global mental health crises, plant medicines have the potential to help billions of people live happier lives. Thanks to university-led and FDA-approved studies, North America is leading the way in advancing an industry as psychedelics are becoming accepted globally for therapeutic, medical, and recreational use. Here are some statistics:
- 32 million people in the U.S. have used psychedelics at least once
- 17% of all American adults between 21 and 64 have used psychedelics at least once
- $500 billion is spent in the U.S. every year on prescription drugs
- $238 billion is spent in the U.S. every year on mental health treatments and ancillary services
- The anxiety disorder and depression treatment market is estimated at $16 billion
- $187.8 billion was spent in 2013 on mental health and substance abuse disorders
Management Team
Delic Co-Founder and CCO Jackee Stang was an executive at High Times, a leading counterculture publication that became the voice for the cannabis industry. The monthly magazine had a circulation of over 500,000 copies per issue. Its website attracted 500,000 to five million users each month by 2014.
Likewise, company Co-Founder and CEO Matt Stang was a previous owner and operator of High Times, a position from which he played an instrumental in legalizing cannabis in multiple states and launched the Cannabis Cup in America. After interacting with the cannabis community for two decades, he helped found Delic in 2019 as one of the first psychedelic corporations. He shapes the company’s vision and path using his expertise in branding, marketing, business development, and product viability.
Delic’s VP of Business Development, John Coleman, Ph.D., is a former president of Anandia Labs, a biotech company focused on genetics and analytics. Having experience in both science and business, Dr. Coleman is well-equipped to lead Delic’s business development efforts as it strives to enter new vertical markets.
Zak Garcia is the company’s Chief Marketing Officer. He was the former CMO of Bulletproof Inc., maker of the well-known Bulletproof Coffee brand. Mr. Garcia is a marketing and leadership strategist who helped grow Bulletproof Coffee to over $250 million in revenue.
DSG Global Inc. (OTCQB: DSGT)
DSG Global Inc. (OTCQB: DSGT) is an emerging global technology company with interconnecting businesses in fast growing market sectors. With roots in the golf industry, the company specializes in golf fleet management and is moving quickly into road-ready electric vehicles for delivery in the third quarter of 2021.
In 2019, the company secured exclusive North America distribution rights for Jonway Automobile Co. road-ready electric vehicles (EVs). Jonway, based in Zhejiang, China, began manufacturing new vehicles s in 2003 and today produces Electric powered Cars, Trucks, Vans, SUV’s, and Scooters. Jonway vehicles are exported to more than 80 countries and are built to comply with U.S. safety and environmental standards.
These vehicles are being sold via DSG’s wholly owned subsidiary, Imperium Motor Company (IMC). The move into consumer vehicles capitalizes on the company’s strength in the selection and distribution of EVs, the ability to work with large manufacturers and in application of proprietary technology unique to DSG. DSG’s advanced fleet tracking can be integrated into Jonway EVs to offer a customized scalable and integrated solution to meet the needs of small businesses and large enterprises.
The Future is Electric
With decades of EV experience in golf, including distribution of highly advanced carts, DSG recognized the huge chasm between consumer interest in acquiring road ready EVs versus current EV models’ lack of availability and affordability. As such, the company focused on becoming a distribution and EV brand management company unencumbered by the manufacturing process. The manufacturers take responsibility for building vehicles to DSG’s specifications and fulfillment of regulatory and licensing requirements.
DSG has also established a distribution agreement with Skywell New Energy Automobile Group Ltd., an Asian-based EV manufacturer. Skywell will supply DSG with SUV’s, Passenger Vans, Cargo Vans, Commercial Vehicles and Buses that will be fully certified for use in the United States.
Brands
Imperium Motor Company (IMC) seeks to transform the way the world drives by making greener transportation available to everyone. IMC is an EV sales and marketing company that distributes directly to consumers and through third party distributors, offering a wide variety of affordable vehicles equipped for the North American market. The company’s emphasis is on great design, a green mindset, performance and functionality. Its vehicles include 26 models of high-speed, mid-speed and low-speed electric vehicles including cars, trucks, SUVs, vans, buses and scooters.
Vantage Tag Systems (VTS) is a global leader in the design, manufacture, and marketing of fleet management solutions for the golf industry. VTS has developed the TAG suite of products that represents the industry’s first completely modular fleet management solution. The company’s patented analytics, mobile touch screen GPS units and electric golf carts are sold around the world through a network of established distributors and partnerships with notable brands in fleet and equipment manufacture. VTS solutions also have applications in managing commercial, agricultural, military and government fleets. VTS is a wholly owned subsidiary of DSG Global.
Market Outlook
The global EV market was valued at $273 billion in 2017, according to Fortune Business Insights, and is projected to exceed $987 billion by 2027, with a projected CAGR of 17.4 percent. The relative high manufacturing costs of EVs compared to gasoline-powered vehicles and the resulting higher sticker price to consumers are major obstacles to near term market adoption.
The global e-bike market is estimated to grow to $70 billion by 2027 from its current valuation of $41.1 billion. An estimated 130 million e-bikes are expected to be sold globally over the next two years. The U.S. imported approximately 600,000 e-bikes in 2020, according to the Light Electric Vehicle Association, and its analysts expect that number will grow substantially in 2021.
Management Team
Robert “Bob” Silzer is the CEO of DSG Global. He is a serial entrepreneur who turns technology ideas in high growth industries into profitable businesses. With roots in the golf industry, he founded Vantage Tag Systems in 2008. Vantage Tag Systems is now a DSG subsidiary specializing in GPS-enabled fleet management.
Zahir Loaiza is the interim CFO of DSG Global. She assumed the role in March 2021, after having previously served as the company’s Corporate Controller. Her diverse international experience includes working at a publicly traded mining company, several law firms and more in the U.S., Canada and South America. Prior to pursuing a career in corporate finance, she was the owner of two retail entities.
Rick Curtis is the president and COO of Imperium Motor Company, the automotive subsidiary of DSG Global. His 40-year background in the automotive industry includes manufacturing, vehicle distribution, parts distribution, service management, dealer development and executive management of dealer groups. Prior to joining Imperium, Mr. Curtis served as president of Mullen Technologies and grew the company into a world class provider of electric vehicles, battery technology and energy storage systems.
William “Bill” Rex is president of Imperium Motor’s EV Bus and Motor Home Division. He has more than 40 years’ experience at suppliers of buses/electric buses, motor homes, trucks, specialty vehicles and batteries. He is the founder of Rexhall Industries Inc., formerly a publicly traded manufacturer of RVs and distributor of buses and coaches. He previously served as president of THOR West, a subsidiary of THOR Industries that manufactures shuttle buses, and as president of BYD Coach and Bus.
Patrick J. Parenti is the SVP Global Sales at DSG subsidiary Vantage Tag Systems. He has nearly 30 years of experience in golf and golf course management. Prior to joining DSG in 2012, Mr. Parenti served for 10 years as SVP at ProLink Systems, a leading global provider of GPS golf-course management systems.
Clint Singer is Director of Engineering at Vantage Tag Systems. He has been a senior developer in the golf industry for more than 20 years and has an extensive background in GPS systems.
Daniel Price is Technical Operations Manager of DSG Global’s European Region, UK, South Africa. In addition to his background in mechanical and electronic engineering, he is an audio engineer, specializing in automotive audio and security. He has also worked with high end electronic security companies in the UK and previously owned an electronic security and CCTV company.
Steven Mueller is Operations Manager at Vantage Tag Systems. He worked in the global pulp and paper market for nine years, facilitating the global movement of thousands of tons of timber products annually. Additionally, he has a successful decades-long track record of managing operations and consulting for a wide range of retail businesses.
Eat Well Investment Group Inc. (CSE: EWG) (OTC: EWGFF)
Eat Well Investment Group Inc. (CSE: EWG) (OTC: EWGFF), headquartered in Vancouver, British Columbia, is a publicly traded vertically integrated plant-based foods company combining the best of agribusiness, foodtech, and CPG brands to supply the world with innovative, delicious, and better-for-you foods. The company supplies Beyond Meat, Ingredion, Nestle, General Mills and more. It is on track to generate $60 million in revenue for 2021 and is projecting $100 million in revenue for 2022.
Eat Well’s management team has an extensive record of sourcing, financing and building successful companies across a broad range of industries and maintains a current investment mandate on the health and wellness industry. The team has financed and invested in early-stage venture companies for more than 25 years, resulting in the ability to construct a portfolio of opportunistic investments intended to generate superior risk-adjusted returns. Eat Well’s strategic advisory board includes pioneers in the plant-based foods industry, including HRH Prince Khaled bin Alwaleed bin Talal Al Saud, Founder and Chief Executive Officer of KBW Ventures, and Jeff Dunn, CEO of Bolthouse Farms who previously held senior leadership positions at both Campbell Soup Company and The Coca Cola Company.
The company’s plant-based investment thesis is centered on growing its seed-to-market operations, which include raw ingredients, processing, pulse fractionation, unique IP and premium consumer packaged goods (CPG). Eat Well Group is building a unique ecosystem that can supply these essential cornerstone needs for society. The company has plant-based foods and nutrition experts specializing in the latest science and original thinking for what consumers want most – high quality and affordability in healthy, clean and simple products.
Eat Well focuses on intellectual property, product portfolio development and long-term value creation for stakeholders in a rapidly expanding industry. As an emergent sector globally, plant-based foods represent a double-digit annual growth category, with more than 35% of the world’s supply of pulse proteins coming from Canada.
Portfolio
On July 31, 2021, Eat Well Group acquired Belle Pulses Ltd., one of the top pulse processors in Canada. Belle Pulses has been operating for over 40 years and had over $60 million in sales in 2020. The company counts a broad range of customers in over 35 countries, including global strategic food companies and major ingredient distributors. Currently, Belle produces nearly 100,000 tons of fully traceable seed and product, yielding over 26,000 tons of pure plant protein.
Eat Well also owns 100% of Sapientia Technology Inc. Led by Dr. Eugenio Bortone – one of the world’s preeminent food scientists and extrusion processing experts and the inventor of Frito-Lay’s Twisted Cheetos – Sapientia has filed four patents around the “protein curl” and crispy-puff-style snack. By focusing on texture and crunch, Sapientia’s patents solve one of the major problems that large scale snack food companies have struggled with for years – how to offer appealing texture and flavor in a guilt-free, not fried, natural and healthy alternative to the majority of snack food products available today.
Eat Well owns a 51% share of Amara Organic Foods, with an option to acquire additional ownership up to 80 percent. Amara, one of the fastest-growing baby food brands in America, is a food technology company that uses science and proprietary IP that locks in taste and texture to make healthy, organic, non-GMO, plant-based, convenient baby and children’s food possible for modern-day families. From baby food to toddler food and beyond, Amara is driven by the belief that setting kids on the right path from a young age will help them live better, feel better and think better for the rest of their lives. Amara’s revenues have grown by more than 400% since January 2021, and the brand’s success has drawn media coverage from business news outlets including Forbes and TechCrunch.
Market Outlook
According to an August 2021 report from Bloomberg Intelligence, the plant-based foods market is expected to experience explosive growth, comprising up to 7.7% of the global protein market by 2030 at a value of over $162 billion, up from $29.4 billion in 2020. Bloomberg notes that plant-based alternatives are here to stay, and that consumption will grow rapidly. Plant-based food sales in 2020 grew twice as fast as overall food sales, according to Polaris Market Research.
Pulse proteins (fava, yellow pea, etc.) are a foundational ingredient to most plant-based foods due to their high protein content and their readily available, affordable supply.
Many analysts view the food tech market as similar to the early days of the Internet in that plant-based foods represent a worldwide secular trend of steady growth and potential that will revolutionize the way society functions and people experience nutrition.
The sector continues to experience significant M&A transactions. Recently, Sol Cuisine was acquired by PlantPlus Foods LLC, a major South American protein producer, in an all-cash transaction valued at approximately $126 million, or 6x revenue.
Management Team
Marc Aneed is President and Director of Eat Well Group. His 20-year career in CPG started at The Quaker Oats Company/PepsiCo, where he worked on iconic brands like Gatorade. He previously was at Glanbia PLC, a global nutrition company, where he led Amazing Grass, a leading plant nutrition and supplement company with over $100 million in retail sales. He also led Glanbia’s Sports Nutrition brands in North America with over $750 million in retail sales. Mr. Aneed has launched dozens of successful consumer products, driving over $1 billion in collective retail sales.
Mark Coles is the company’s Chief Investment Officer. He is a veteran CPG senior executive specializing in the plant-based foods sector. For the past decade, Mr. Coles has spearheaded global plant-based start-up initiatives, culminating in a 2020 acquisition by an international New York Stock Exchange-listed food ingredient company. He has over 25 years of experience in CPG-focused strategy, mergers and acquisitions and project financing.
Patrick Dunn is Eat Well Group’s Vice President, Finance. He is the founding partner of Dunn, Pariser & Peyrot and has a track record of building highly successful agribusinesses throughout North America and other international markets. As a testimony to his business portfolio work, Mr. Dunn and his firm have won multiple industry awards for accounting, finance and business management.
Barry Didato is the company’s Vice President, Strategy. He is focused on the development of strategic revenue channels, sales partnerships, and international distribution for Eat Well Group. Mr. Didato brings extensive strategic sales capabilities and an extensive network of contacts in the industry to the company. Prior to joining Eat Well Group, he served for over 18 years as a senior advisor for several ultra-high net worth family offices and numerous innovative wellness, nutrition, medical, and food businesses.
Strategic Advisory Board
HRH Prince Khaled bin Alwaleed bin Talal Al Saud, Founder and Chief Executive Officer of KBW Ventures, is a firm supporter of clean energy and the humane treatment of animals. He is also a vocal supporter of the private sector in the Middle East. A member of the Saudi Arabian Royal Family, Prince Khaled was born in Stanford and spent his youth in Riyadh under the mentorship of his father, philanthropist HRH Prince Alwaleed bin Talal Al Saud, Chairman of Kingdom Holding Company. He is also the Founding Chairman of KBW Investments and serves across several boards. He invests in an array of successful but diverse global businesses – from promising technology startups to established companies. Today, with holdings on three continents, Prince Khaled stands at the gateway between the Middle East’s evolving economies and the Western world. Consistently, Prince Khaled’s focus is on ventures and ideas at the intersection of innovation and economic growth.
Jeff Dunn has over 30 years of experience in agriculture and packaged food, including senior leadership positions with Bolthouse Farms, Campbell Soup Company and The Coca Cola Company, among others. He is an Operating Partner at Butterfly and focuses primarily on the agriculture & aquaculture and food & beverage product sectors. Prior to joining Butterfly, Mr. Dunn was the President of the Campbell Fresh division of Campbell Soup Company from 2015 to 2016, where he was in charge of building Campbell’s scale and accelerating its growth in the rapidly expanding packaged fresh segments and categories across the retail perimeter.
Energy Fuels Inc. (NYSE American: UUUU) (TSX: EFR)
Energy Fuels Inc. (NYSE American: UUUU) (TSX: EFR), based in Lakewood, Colorado, is the country’s largest producer of uranium and the leading conventional producer of vanadium, both designated by the U.S. government as critical minerals.
As the leading U.S. diversified uranium miner, Energy Fuels’ uranium production portfolio stands apart in the world. Energy Fuels has more uranium production facilities, more production capacity, and more in-ground resources than any other company in the United States. In fact, the company’s assets have produced over one-third of all U.S. uranium over the past 15 years and is uniquely positioned to increase production to meet new demand.
Energy Fuels utilizes both conventional and in-situ recovery (“ISR”) technology to produce uranium from three strategic facilities:
- White Mesa Mill in Utah (conventional) has a licensed capacity of over 8 million pounds of U3O8 per year. The highly strategic White Mesa Mill is the only conventional uranium mill in the country and is proximate to some of the largest and highest-grade uranium mines and projects in the U.S., including the Company’s Canyon mine, La Sal Complex, Henry Mountains Complex and Roca Honda Project. White Mesa Mill provides Energy Fuels with significant production scalability as uranium demand increases. The White Mesa Mill also has other diverse businesses, including vanadium, rare earth elements (REE’s), alternate feed materials recycling and land cleanup, all described below.
- Nichols Ranch Plant (ISR) is located in the productive Powder River Basin district of Wyoming and has a total licensed capacity of 2 million pounds of U3O8 per year. Nichols Ranch has produced 1.2 million pounds of U3O8 since commissioning in 2014, and it has significant future expansion potential from 34 fully licensed wellfields containing significant in-ground uranium resources.
- Alta Mesa Plant (ISR) is located on over 200,000 acres of private land in Texas. The fully licensed and constructed ISR project has a total operating capacity of 1.5 million pounds of uranium per year and produced nearly 5 million pounds of U3O8 between 2005 and 2013. This low-cost production facility is currently on standby, maintained in a state of readiness to respond to expected increases in demand.

In addition to being the largest uranium miner in the U.S., Energy Fuels’ overall portfolio also includes a pipeline of high-quality, large-scale exploration and development projects that are permitted or are in advanced stages of permitting, as well as an industry-leading U.S. NI 43-101 Mineral Resource portfolio.
FACTOID: Energy Fuels has led industry efforts over the past two-plus years to get the U.S. government to recognize the importance of domestically produced uranium, including the 2018 – 2019 Uranium Section 232, the ongoing Nuclear Fuel Working Group and the recently announced creation of the U.S. strategic uranium reserve. The U.S. is by far the largest consumer of uranium in the world, yet we import almost all of our requirements; Energy Fuels aims to change that.
Nuclear Market Potential
Multiple studies in top scientific journals have shown that nuclear power is cleanest and most economical way to produce reliable electricity as worldwide demand continues to soar. Nuclear power is presently the only available and affordable low-carbon power source that can meet both current and future baseload electricity demands while simultaneously reducing air pollution and mitigating climate change. U.S. nuclear power plants currently generate nearly 20% of the nation’s electricity overall and 55% of its carbon-free electricity and even a modest increase in electricity demand would require significant new nuclear capacity by 2025. According to the World Nuclear Association (WNA), there are currently 441 operable reactors, with another 54 units under construction and 439 in various stages of planning; in addition, the WNA has identified a potentially massive supply/demand gap through 2040 of 1 billion pounds. These factors among others are expected to significantly drive increased demand for uranium.
Reasons Nuclear is Gaining Traction
- Nuclear reactors emit no greenhouse gases during operation. Over their full lifetimes, they result in comparable emissions to renewable forms of energy such as wind and solar.
- Unlike any other form of energy, the waste from nuclear energy is contained and managed securely. Used fuel is currently being safely stored for ultimate disposal or future reprocessing, and 96% of this waste can potentially be recycled.
- Greater demand for clean electricity to power everything from homes to automobiles, reducing dependence on fossil fuels.
No. 1 U.S. Producer of Vanadium in 2019

Energy Fuels also produces vanadium as a byproduct of uranium production. Vanadium is designated a critical mineral, essential to the economic and national security of the United States. Energy Fuels was the largest producer of vanadium in the U.S. in 2019, and has significant high-grade, in-ground vanadium resources, as well as a separate high-purity vanadium production circuit at their White Mesa Mill, which is also the only conventional vanadium mill in the country. Crucial for use in the steel, aerospace, and chemical industries, vanadium plays a critical role in the production of high-strength and light-weight metallic alloys and demand is expected to increase across the globe.
Energy Fuels has several fully permitted and developed standby mines containing large quantities of high-grade vanadium, along with uranium, including:
- La Sal Complex (Utah)
- Whirlwind Mine (Colorado/Utah)
- Rim Mine (Colorado)
Vanadium has also gained increased attention as a catalyst in next-generation high-capacity, “community-scale” batteries used for energy storage generated from renewable sources. Demand is only expected to grow as this market expands. With recent upgrades in its vanadium production operations, in 2019 Energy Fuels produced commercial levels of the highest purity (99.7%) vanadium in the mill’s history and can rapidly adjust production to meet volatile market conditions. Energy Fuels is one of the very few known avenues that provides investors access the vanadium market.
Rare Earth Element (REE) Production, Alternate Feed Material Recycling, and Land Cleanup
The White Mesa Mill also provides the company with diverse cashflow generating opportunities. Security of supply for Rare Earth Elements (REEs) supporting U.S. military and defense requirements is a major issue today. Energy Fuels has been approached by a number of entities, including the U.S. government, inquiring about the potential to process certain REEs at the mill. The White Mesa Mill is currently licensed to process certain REEs, including tantalum and niobium. And, early indications are that the mill can be utilized to produce several other REEs. The White Mesa Mill is also the only facility in North America licensed and capable of recycling alternate feed materials (AFMs). AFMs are essentially low-level waste materials that contain recoverable quantities of natural (or unenriched) uranium. The Company typically generates between $5 and $15 million per year from AFM recycling. Finally, Energy Fuels is seeking to become involved in the cleanup of legacy Cold War era uranium mines in the Four Corners region of the U.S., including on the Navajo Nation. The U.S. Environmental Protection Agency (EPA) has access to over $1.5 billion for the cleanup of just a fraction of the sites on the Navajo Nation. The White Mesa Mill is fully licensed to receive much of this material, we are one of the government’s lowest cost options, and we have the ability to recycle the material and produce usable uranium from it.
Management Team
Mark S. Chalmers, President and CEO
Mark S. Chalmers is the president and chief executive officer of Energy Fuels, a position he has held since Feb. 1, 2018, following his role as chief operating officer of Energy Fuels from July 1, 2016 – Jan. 31, 2018. From 2011 to 2015, Chalmers served as executive general manager of Production for Paladin Energy Ltd., a uranium producer with assets in Australia and Africa, including the Langer Heinrich and Kayelekera mines where, as head of operations, he oversaw sustained, significant increases in production while reducing operating costs. He also possesses extensive experience in in situ recovery (“ISR”) uranium production, including management of the Beverley Uranium Mine owned by General Atomics (Australia), and the Highland mine owned by Cameco Corporation (USA). Chalmers has also consulted to several of the largest players in the uranium supply sector, including BHP Billiton, Rio Tinto, and Marubeni, and until recently served as the chair of the Australian Uranium Council, a position he held for 10 years. Chalmers is a registered professional engineer and holds a Bachelor of Science in Mining Engineering from the University of Arizona.
W. Paul Goranson, COO
W. Paul Goranson is the chief operating officer for Energy Fuels. Goranson has 30 years of mining, processing and regulatory experience in the uranium extraction industry that includes both conventional and in-situ recovery (“ISR”) mining, and he is a registered professional engineer. Prior to the acquisition by Energy Fuels of Uranerz Energy Corporation, Goranson served as president, chief operating officer and director for Uranerz, where he was responsible for operations of the Nichols Ranch ISR Uranium Project. In addition to those duties, he also managed uranium marketing, regulatory and government affairs, exploration and land. Prior to joining Uranerz, Goranson served as president of Cameco Resources, where he led the operations at the Smith Ranch-Highland, Crow Butte and North Butte ISR uranium recovery facilities. Goranson also served as vice president of Mesteña Uranium LLC, and he has served in senior positions with Rio Algom Mining, (a subsidiary of BHP Billiton), and Uranium Resource Inc. Goranson has a Bachelor of Science in Natural Gas Engineering from Texas A&I University, and a Master of Science in Environmental Engineering from Texas A&M University-Kingsville.
David C. Frydenlund, CFO, General Counsel, Corporate Secretary
David C. Frydenlund is chief financial officer, general counsel, and corporate secretary of Energy Fuels. His responsibilities include oversight of all legal matters relating to the company’s activities. His expertise extends to NRC, EPA, state and federal regulatory and environmental laws and regulations. From 1997 to 2012, Frydenlund was vice president of regulatory affairs, general counsel and corporate secretary of Denison Mines Corp., and its predecessor International Uranium Corporation (“IUC”). He also served as a director of IUC from 1997 to 2006 and CFO of IUC from 2000 to 2005. From 1996 to 1997, Frydenlund was vice president of the Lundin Group of international public mining and oil and gas companies, and prior thereto was a partner with the Vancouver law firm of Ladner Downs (now Borden Ladner Gervais) where his practice focused on corporate, securities and international mining transactions law. Frydenlund holds a bachelor’s degree in business and economics from Simon Fraser University, a master’s degree in economics and finance from the University of Chicago and a law degree from the University of Toronto.
Curtis H. Moore, Vice President of Marketing and Corporate Development
Curtis H. Moore is the vice president of Marketing and Corporate Development for Energy Fuels. He oversees product marketing for Energy Fuels, and is closely involved in mergers & acquisitions, investor relations, public relations, and corporate legal. He has been with Energy Fuels for over 12 years, holding various roles of increasing responsibility. Prior to joining Energy Fuels, Moore worked in multi-family real estate development, government relations and public affairs, production homebuilding, and private law practice. Moore is a licensed attorney in the State of Colorado. He holds Juris Doctor and MBA degrees from the University of Colorado at Boulder, and a Bachelor of Arts dual degree in Economics-Government from Claremont McKenna College in Claremont, California.
EverGen Infrastructure Corp. (TSX.V: EVGN) (OTCQB: EVGIF)
EverGen Infrastructure Corp. (TSX.V: EVGN) (OTCQB: EVGIF) is developing Canada’s Renewable Natural Gas Infrastructure Platform, starting on the west coast in British Columbia. The company is combating climate change and helping communities contribute to a sustainable future by acquiring, developing, building, owning and operating a portfolio of renewable natural gas (RNG), waste-to-energy, and related infrastructure projects.
While EverGen is currently focused on British Columbia, its continued growth is expected across other regions of North America. RNG is produced differently than conventional natural gas, without drilling wells. RNG is derived from biogas, which is captured from decomposing organic waste in landfills, food waste, agricultural waste matter and wastewater from treatment facilities. This waste feedstock is supplied to an anaerobic digester which contains bacteria that breaks down organic matter in the absence of oxygen. The resulting biogas is captured and cleaned to create carbon neutral or carbon negative RNG to be used by the existing North American gas pipeline grid. By capturing these emissions and transforming them into RNG, then combusting into CO2, the overall greenhouse gases (GHG) impact is materially less potent than allowing natural decomposition to release methane into the atmosphere. Liquid and solid digestate matter is a byproduct of the RNG production process and is used as fertilizer and in other applications.
EverGen operates three projects in British Columbia. The company was incorporated in 2020 and went public in 2021, with its common shares listed on the TSX Venture Exchange under ticker symbol ‘EVGN’. In February 2022, EverGen’s common shares began trading on the OTCQB Venture Market in the U.S. under ticker symbol ‘EVGIF’. The company is headquartered in Vancouver.
Portfolio Projects
Fraser Valley Biogas is one of three projects in EverGen’s portfolio. Located in Abbotsford, British Columbia, the facility has been digesting manure and off-farm organics since 2011 and was the first agricultural digester in Canada to produce RNG. The RNG generated through this project is part of a FortisBC program to supply renewable gas to homes, businesses and other customers. Fraser Valley Biogas also provides Abbotsford farms with renewable fertilizer via the digestate produced. EverGen acquired Fraser Valley Biogas early in 2021 and is currently enhancing and expanding the facility. These optimization projects resulted in record production during the month of September 2021, supporting the growing demand for RNG in British Columbia. Optimization activities contributed an additional 18% of RNG production for September and a 9% higher year-to-date production compared to the previous year. The facility produces approximately 80,000 gigajoules of RNG, enough to heat more than 1,000 homes for a year.
Net Zero Waste Abbotsford, a wholly owned EverGen subsidiary and portfolio project, is an existing composting and organic processing facility and RNG expansion project. The British Columbia Utilities Commission recently approved a 20-year offtake agreement between the facility and FortisBC, an electricity and gas utility. Under this agreement, FortisBC will purchase up to 173,000 gigajoules of RNG annually for injection into its natural gas system upon completion of an anaerobic digester project at Net Zero Waste Abbotsford. Once construction is complete, this project is expected to produce enough energy to meet the needs of more than 1,900 homes.
Sea to Sky Soils, a wholly owned EverGen subsidiary and portfolio project, is an existing composting and organic processing facility and potential future RNG expansion project which has been operating near Pemberton, British Columbia, on Lil’wat Nation land since 2012. The Lil’wat Nation is a key partner and supporter of the facility, which has employed a majority of its staff from the First Nation since inception. The Sea to Sky Soils facility processed approximately 160 percent of its forecast tonnage in the second half of 2021. In total, Sea to Sky Soils processed approximately 36,000 tons of organic waste in 2021. The facility is working with the Ministry of Environment to expand its operational capacity in 2022. EverGen has partnered with local municipalities – including Metro Vancouver and the municipality of Pemberton – for the delivery of additional organic waste to the facility. The facility is an important part of EverGen’s RNG infrastructure platform and serves as a source of valuable feedstock to support the company’s existing and future operations.
Market Outlook
A report from Global Market Insights states that the biogas market is projected to see significant growth over the next few years, driven by a shifting preference to utilize biogas to reduce emission levels from traditional fuels. Escalating RNG usage by gas utilities as a sustainable and low carbon alternative to supply heat and electricity in industries and buildings will further stimulate growth. RNG is increasingly deployed across the transport sector, especially for heavy vehicles and vessels, to abate GHG emissions.
Many North American gas utilities have set RNG targets of 5% to 15% of production by volume in 2030, compared to less than 1% by volume in 2020. FortisBC has a goal of including 15% RNG in its gas supply by 2030. EverGen believes this presents a potential C$16 billion+ opportunity for RNG producers.
Management Team
Chase Edgelow is co-founder and CEO at EverGen. He has over 15 years of specialized private investment, finance, and technical expertise in the energy and infrastructure sectors. His background is as a Facilities Engineer with Petro-Canada, independently managing energy infrastructure capital projects located in western Canada. He holds a Professional Engineer designation from the province of Alberta.
Mischa Zajtmann is co-founder and President at Evergen. He has 15 years of experience providing consulting and management for Canadian and American companies in the natural resources and energy space. He is a corporate securities lawyer who began his career at Blake, Cassels & Graydon LLP. His J.D. is from the University of Saskatchewan Law School. He’s a member of the British Columbia Bar.
Sean Mezei is COO at EverGen. He has 20 years of experience in the RNG industry, having served previously as the president of Greenlane Biogas and as a senior manager at QuestAir, and founder and president of Dekany Consulting. He was a co-chairman of the American Biogas Council’s RNG working group for six years. He has been a Registered Professional Engineer in the province of British Columbia since 1994.
Natasha Monk is CFO at EverGen. She is a CPA with 12 years accounting, financial reporting, and tax experience in public practice and industry. She is currently a partner at Affirm LLP, where she advises and consults to a wide variety of companies in multiple industries across public and private sectors. Prior to joining EverGen, she worked at KPMG. She graduated from the University of Calgary.
Exro Technologies Inc. (TSX: XRO) (OTCQB: EXROF)
Exro Technologies Inc. (TSX: XRO) (OTCQB: EXROF), a Canadian technology company, is an innovative pioneer in the energy sector. Exro has developed and commercialized an electric power module (EPM) that integrates into existing motor systems to make them smarter. Exro’s patented technology optimizes existing motor performance by automatically sensing and adapting operating parameters to an optimized state, creating measurable efficiency gains, reduced mechanical components and increased system availability.
Applications
Exro’s technology and efficiency optimization algorithms improve the performance and efficiency of electric motors by manipulating power delivery to individual coils, thereby enabling the ability to expand operating parameters. This novel approach is scalable and can be utilized in most variable torque applications.
The widespread applications of Exro’s technology apply to optimizing the performance of electric vehicles, locomotive traction applications, industrial motors, and other variable torque applications that benefit from smart energy conversion.
Intellectual Property
Exro’s proprietary, patented software controls electric motor coils through individual coil switching. This introduction of intelligence into energy conversion at the level of individual coils results in expanded speed/torque capability, improved machine efficiency, reliability, safety and maintenance across a wider operating range. Exro’s advanced control algorithms create smart, real-time optimized power management.
Exro currently holds 15 patents, with 8 patents pending and additional patents under development. The company continues to expand its IP portfolio to support its goal of becoming a globally recognized leader in leveraging advanced control algorithms to improve the performance, efficiency and longevity of electric motors and generators.
Market Opportunity
Electric motors are the single biggest consumer of electricity. They account for about two-thirds of industrial power consumption and about 45% of global power consumption, according to an analysis by the International Energy Agency. Exro’s technology seeks to give industries a new way to look at energy—from electric vehicles, to industrial equipment, to renewable applications like wind farms; we are improving the way energy is consumed.
Laboratory Expansion
The 6,500-square-foot Exro Innovation Center (EIC), scheduled to open spring of 2020 in Calgary, will transition the current Victoria lab into one Calgary based center. The company’s new laboratory space will expand its service capabilities to customers, provide larger test capabilities, and showcase how Exro’s technology can be applied to dramatically improve the performance of electrical motors.
The EIC will also host collaborative events to explore advances in energy consumption and electric motor innovations, with participants from across Canada and around the world.
Strategic Partnerships
- A strategic agreement with Finland’s Aurora Powertrains Oy, which in 2019 released an all-electric production snowmobile called the “eSled,” will see Exro’s technology added to the Aurora electric powertrain. The snowmobile sector’s economic footprint is estimated at $26 billion in the U.S., $8 billion in Canada, and $5 billion in Europe and Asia.
- An agreement with Potencia in Mexico serving the last mile vehicle segment will integrate Exro’s custom drive and EPM module into small passenger commercial vehicles (taxis) and fleet delivery trucks
- A licensing agreement with Motorino Electric, a leader in the Canadian electric transportation industry, will integrate Exro’s Electric Power Module technology into Motorino’s CTi electric bicycle.
Management
Chief Executive Officer Sue Ozdemir is a proven leader in the innovation and manufacturing of electric motors. She has nine years of accomplishments at General Electric, acting as CCO and the CEO of GE’s Small Industrial Motors Division, overseeing the division’s North American and international markets – ultimately building the division into a $160 million enterprise.
Chief Commercial Officer Josh Sobil is leading the seamless adoption of Exro’s growing product portfolio focused on the mobility segment and opening doors in all segments including agriculture, heavy industry, energy, construction, among others.
Executive Chairman Mark Godsy is a serial technology entrepreneur who has been involved in many top tier ventures, including two of Canada’s most successful biotech companies.
FingerMotion Inc. (NASDAQ: FNGR)
FingerMotion Inc. (NASDAQ: FNGR) is an evolving technological company with core competencies in mobile payment and recharge platform solutions in China. FingerMotion is in the process of developing additional value-added technologies to market to users.
Founded in 2016, FingerMotion’s goal is to serve over a billion users in the Chinese market and expand its model to other regional markets. The company has offices in Hong Kong, Shanghai and New York City.
Current Offerings
FingerMotion is analyzing and transforming mobile data to improve the lifestyle of the public through technology and innovation. The company’s current offerings include:
- Telecommunications Products and Services – FingerMotion’s proprietary universal exchange platform, ‘PigeonHole Integration System (PIS)’, offers seamless integration between telecom operators and online stores. The service platform’s offerings include top up and recharge, data plan, mobile phone, loyalty points redemption and subscription plans. The platform offers reliable and secure transactions, real-time reconciliation, simple integration for partners and efficient settlements.
- SMS and MMS Services – The integrated platform is registered as FingerMotion’s IP in China and provides a robust back-end control panel for corporate partners to manage their own messaging settings. FingerMotion’s clients range from insurance to financial industries, ecommerce firms, airlines and more. The platform offers competitive pricing for partners and provides quick and efficient review to meet timely marketing initiatives.
- Big Data Insights – FingerMotion brings Big Data-enabled insurance solutions through its Big Data Insights arm, Sapientus. The company’s strategic partnerships with the largest Chinese telecommunications giants allow access to uncover behavior insights through geolocation and mobile data usage. Its Big Data offerings include risk scoring, precise marketing, simplified underwriting and customized products.
- Rich Communication Services (RCS) – FingerMotion’s RCS platform will be a proprietary business messaging solution that enables businesses and brands to communicate their services to customers via 5G infrastructure. The company expects its RCS platform to offer a better user experience, more efficiency and cost-effectiveness when compared to other solutions.
Telecommunications and Insurtech Markets
The global telecommunications market was valued at $1.74 trillion in 2019 and is expected to grow at a CAGR of 5% from 2020 to 2027. The steady increase is expected to be driven by the adoption of 5G and the increased popularity of Internet of Things (IoT) applications.
The Chinese telecom market was valued at $254.1 billion in 2017 and is also constantly expanding. The current Chinese telecom market is dominated by three mobile operators – China Mobile, China Unicom and China Telecom, which together are responsible for around 1.6 billion active subscribers (https://ibn.fm/zfwy9).
In addition, the insurtech (insurance technology) market was valued at $2.72 billion globally in 2020 and is expected to grow at a CAGR of 48.8% from 2021 to 2028. The large increase is attributed to the rising use of technology solutions for everyday activities like acquiring insurance coverage (https://ibn.fm/TGo7D).
Through its proprietary platforms and technologies, FingerMotion is uniquely positioned to capitalize on the telecom and insurtech markets’ growth and opportunities.
Management Team
Martin J. Shen is the Chief Executive Officer of FingerMotion Inc. He has over 15 years of experience in senior management roles within entrepreneurial startups and large multinational corporations. He has acquired a wide range of corporate management, financial oversight and operation administration expertise through these roles. In his most recent role, he founded Imperial Distributors (formerly known as AP Martin Pharmaceutical Supplies Ltd.), establishing the company as the preferred choice for distributional support to regional pharmacies throughout Western Canada. Before founding Imperial, Mr. Shen served as the Chief Operating Officer and Chief Financial Officer at Wales and Son Industrial (formerly Weir Minerals), a firm specializing in global delivery and support for mining slurry equipment. He began his career at PricewaterhouseCoopers in Vancouver, with work tours in the tax department in Singapore and the tax audit and advisory group in Hong Kong. Mr. Shen is a U.S. Certified Public Accountant and holds a Bachelor of Science from the University of British Columbia.
Lee Yew Hon is the company’s Chief Financial Officer. From 2006 until November 2020, he was the Chief Financial Officer of Cubinet Interactive Group of Companies, and he also took on the Chief Operating Officer role in 2011. During his tenure, he was instrumental in leading Cubinet and building teams across the Southeast Asia region, setting up financial processes within a short time. Mr. Lee spearheaded the growth of Cubinet to other regions, including Europe, the Middle East and Russia. He received his diploma from Tunku Abdul Rahman College in 1996. He is a Chartered Accountant, a member of the Malaysia Institute of Accountants (MIA) and an Associate Member of the Chartered Institute of Management Accountants, UK (ACMA).
Li Li is the Senior Vice President of FingerMotion. She recently served as Advisor to Shenzhen WuYiKa Technology Co. Ltd., a comprehensive service platform dedicated to online service distribution and payment. The company has become a fast and efficient provider of new media marketing solutions for the mobile internet. She has held high-level management positions with multiple industry names, including Hangzhou JiuYue Information Technology Co. Ltd. and Hangzhou LingXuan Information Technology. Ms. Li started her career in 2004, founding Shanghai ChuangYeZZ Network Technology Co. Ltd. and serving as its Vice President. With the close cooperation of local operators, the company launched SMS, MMS, WAP, mobile JAVA games, Hunan Satellite TV e-magazine and other wireless internet services to meet the rapid development of wireless internet and application requirements. She received her degree from Nanjing Academy of Engineering.
First Energy Metals Ltd. (CSE: FE) (OTCQB: FEMFF)
First Energy Metals Ltd. (CSE: FE) (OTCQB: FEMFF) is a publicly traded Canadian mineral exploration company. Its primary focus is on developing a multi-commodity mineral property portfolio by identifying, acquiring and exploring North American mineral prospects in the precious metal, base metal and industrial metals sectors.
Headquartered in Vancouver, the company (formerly known as “Agave Silver”) was first incorporated on October 12, 1966.
Core Properties – Augustus Lithium and Titan Gold
Located in Landrienne & Lacorne-Townships, Quebec, Canada, in an active lithium exploration/mining area, the Augustus Lithium Property and surrounding claims total 14,367.71 hectares . It is equipped with excellent infrastructure support, including a road network, railway, electricity, water and trained manpower available locally.
Other highlights of the Augustus Lithium Property include:
- Geologically similar to Sayona Mining’s Authier Lithium project and Mine Quebec Lithium project located 6-12 km away.
- Documented historical drilling over 10,000m in 62 drill holes, worth over $2 million in present day exploration expenditures.
- Two prominent lithium and one silver prospects located on the property.
- A potential high grade lithium resource target of 4 million tonnes at 1% lithium oxide (Li2O).
- Potential for large volume low grade bulk tonnage near surface.
- Two phase exploration work program includes: data compilation, geological mapping, trenching and sampling in Phase 1 (estimated cost $191,418) and diamond drilling, metallurgical testing and resource estimation in Phase 2 (estimated cost $1,166,963).
The Titan Gold Property is located in the Detour-Fenlon Greenstone Belt of east-central Quebec and is comprised of 80 mining claims totaling 4,334 hectares.
Other highlights of the Titan Gold Property include:
- The Detour-Fenlon Greenstone Belt is host to the Detour Mine containing 20 million ounces of gold. The Fenlon Project of Wallbridge Mining has also reported strong high-grade gold intercepts and a successful high-grade (18.49 g/t Au) bulk sample.
- Hosted within a structurally active geological environment with several northwest trending deformation zones which are splays off the Sunday Lake Deformation Zone – all key ingredients to the gold mineralization in the area.
- The property has seen little historical exploration yet sits within what is becoming a prolific recognized gold camp.
Non-Core Properties – Kokanee Creek Gold and Scramble Mine Properties
The Kokanee Creek Gold Property consists of three mineral claims covering approximately 1,590.29 hectares in the Nelson Mining Division in British Columbia.
Other highlights of the Kokanee Creek Gold Property include:
- Gold mineralization indicated in surface samples from historical work since 1979.
- Subsurface gold mineralization discovered in drill holes.
- Continuity of mineralized zones indicated through geological mapping, geochemical and geophysical survey.
- Past producing mines in the vicinity, including the Molly Gibson and the Alpine deposits.
- Historical production reported for the Molly Gibson Mine from 1909-1940 was at an average grade of 36.1 g/t Au and 15.3 g/t silver, with recent exploration returning samples running up to 270 g/t Au.
- Revived exploration on the Alpine deposit area has reported a 2018 inferred resource of 142,000 oz at 16.52 g/t Au using a cut-off grade of 5.0 g/t.
First Energy Metals also holds an option to acquire a 100% interest in the Scramble Mine Gold property, located approximately 8 km east of the town of Kenora in Northwestern Ontario. The mine was discovered in 1894 but remained essentially dormant until 1984, when Boise Cascade Canada Ltd. commenced an evaluation of the property. Since 1984, approximately 5,200 meters of diamond drilling, 250 meters of surface stripping with sampling and 450 meters of underground development have taken place at the property.
Other highlights of the Scramble Mine Property uncovered as part of the company’s 2020 prospecting and sampling programs include:
- Average value of gold in surface samples is 29.34 grams per tonne (1.03 ounces per tonne).
- Gold assays ranged from 5.03 grams per tonne (0.18 oz/t) to 82.30 grams per tonne (2.90 oz/t), with two samples assayed over 2 oz/t.
- All samples assayed over 5 grams per tonne gold.
Market Outlook
The global precious metals market was valued at $193.3 billion in 2020 and is expected to grow at a CAGR of 9%, resulting in a market valuation of $362.1 billion by 2027 (https://ibn.fm/WvN9Z).
The global lithium metal market was valued at $534.6 million in 2020. Through 2027, it is expected to grow at a CAGR of 9.6%, resulting in a forecast valuation of $926.6 million (https://ibn.fm/xBXcx).
First Energy Metals is well positioned to leverage growth opportunities in these expanding sectors through exploration of both its core and non-core properties.
Management Team
Gurminder Sangha is the Chief Executive Officer and Director of First Energy Metals Ltd. He is experienced in the financial industry, focusing on providing advisory-level services to privately and publicly traded companies. Mr. Sangha brings over 18 years of diverse experience related to financial management, business leadership and corporate strategy to his role with First Energy Metals. During his tenure as a board member for various publicly traded companies, he has led initiatives related to corporate finance, business development and corporate governance. Mr. Sangha has an MBA from both Queens University and Cornell University.
Jurgen Wolf is the Chief Financial Officer and Corporate Secretary for First Energy Metals Ltd. He has been involved in the oil and gas industries for over 15 years, assisting public companies with administration and investor relations. Mr. Wolf was educated in Germany and immigrated to Canada in 1953. From 1958 to 1982, he owned and operated pre-cast concrete factories in Calgary and Vancouver. From 1982 to 2002, Mr. Wolf owned and operated J.A. Wolf Projects Ltd., a commercial construction company. He is the previous President and Director of the former US Oil and Gas Resources Inc., which amalgamated to form Petrichor Energy Inc. in 2005. Mr. Wolf retains director roles with several public companies.
Flora Growth Corp. (NASDAQ: FLGC)
Flora Growth Corp. (NASDAQ: FLGC) is an internationally focused cannabis brand builder that leverages natural, cost-effective cultivation practices to supply cannabis derivatives to its diverse business divisions, including cosmetics, hemp textiles, and food and beverage. Flora Growth operates one of the largest outdoor cultivation facilities in the world with an aim of marketing a higher-quality premium product at below-market prices. By prioritizing natural ingredients and value-chain sustainability across its portfolio, the company creates premium products that help consumers restore and thrive.
Flora Growth completed the first traditional cannabis IPO on Nasdaq in May 2021. Although currently headquartered in Toronto, Ontario, with plans to relocate its head office to Miami, Florida, the company’s base of operations is in Colombia, where it has built an extensive distribution network that includes Colombia’s largest distributors.
Currently, Flora Growth is organically growing market share for its existing brand portfolio (pharmaceuticals, textiles, cosmetics, and food & beverage) while seeking revenue-generating acquisitions that offer an accretive distribution network to amplify revenue growth.
Existing Brand & Product Portfolio
Flora Growth’s portfolio spans a number of verticals – each with a thoughtful brand designed to resonate with its intended end consumer. In line with the company’s mission, each brand prioritizes natural ingredients and value-chain sustainability.
Flora Lab S.A.S
Flora Lab is the company’s GMP certified manufacturing and R&D center focused on producing pharmaceuticals, cosmetics, and nutraceuticals for domestic and international markets. Its offerings include product lines that are private label, white-label, and custom formulas.
Through Flora Lab, Flora Growth has relationships with 1,500+ distribution channels, manufactures 63+ OTC products registered with INVIMA (Colombia National Food and Drug Surveillance Institute), and holds multiple GMP certifications enabling international export in an effort to leverage Flora Lab’s capacity to produce a wide range of CBD-infused products.
Flora Beauty
Flora Beauty is the company’s CBD beauty and cosmetics division founded by fashion and beauty industry icon Paulina Vega. Its current offerings include two CBD skincare brands targeting the U.S. and Latin American markets – MIND NATURALS and AWE. These lines exemplify Flora Growth’s socially conscious approach to business.
Currently, Flora Beauty products are offered globally through e-commerce, as well as through Falabella’s 111 retail locations across Latin America. The company is in negotiations with major department stores to launch the line in the U.S. and is also exploring opportunities in the U.K. and other European markets.

KASA Wholefoods
KASA Wholefoods is a Colombian manufacturer of food and beverages leveraging responsibly sourced exotic fruits from the Amazon. KASA has a $10 million+ distribution agreement with Tropi, Colombia’s largest food distributor, which has 130,000+ distribution points across the country.
Mambe, KASA’s leading brand, is already offered through over 980 distribution points across Colombia. Flora Growth expects this network to grow to over 1,200 distribution points in 2021, including one of Colombia’s largest coffee chains, Tostao Café & Pan.

Hemp Textiles & Co.
Through its Hemp Textiles division, Flora Growth intends to utilize its large land package and cultivation infrastructure to capture market share in the rapidly growing hemp industrials segment.
The company’s first brand through this division, Stardog Loungewear, offers a line of comfortable loungewear made from natural, organic materials. Stardog has been distributing globally through e-commerce and brick and mortar channels in Bogota since fall 2020, and the company intends to open U.S. brick and mortar locations in 2021.

Accretive M&A
Flora Growth is targeting transactions to complete the supply chain via key infrastructure to enhance its global distribution with the aim to compete on low-cost, high-quality inputs paired with premium brands that create business lines with robust margins.
To date, Flora has announced two major transactions.
Koch & Gsell (Acquisition)
- Amplify CPG portfolio’s revenue growth through leading brand, Heimat, currently with TTM revenues of $7.6 million.
- Leverage Koch &Gsell’s distribution network of 2,500+ stores to introduce Flora to the Swiss, European and Asian markets.
- Bring patented hemp cigarette manufacturing technology into new markets utilizing Flora’s high-quality cannabis.
Hoshi International (Investment)
- Equity Investment of €2 million into Hoshi to establish Flora as a preferred supplier to two EU processing facilities.
- Opens gateway for Flora Growth’s cannabis through international distribution agreements in the EU and U.K.
- Hoshi’s experienced team and increased access to the EU cannabis market to serve as a catalyst for revenue growth.
Cultivation
Key to Flora Growth’s expansion efforts is its cultivation strategy. The company’s Cosechemos farm, located in Bucaramanga, Colombia, is currently licensed to cultivate 247 acres of cannabis. Through three successful pilot crop plantings, the location has demonstrated a production cost of just $0.06/gram. For comparison, the average cost of North American cannabis (based on 2019 figures from Aphria, Tilray, Sundial, and Aurora) equates to roughly $1.89/gram.
Flora Growth is uniquely positioned to capitalize on Colombia’s favorable growing conditions, low-cost infrastructure, and affordable local workforce as it looks to ramp up its cultivation efforts moving forward.

Leadership Team
Bernard Wilson is the Chairman of Flora Growth. A senior financial professional, Dr. Wilson is the former Vice-Chairman of PricewaterhouseCoopers LLP and is the Chairman of the Founders Board of the Institute of Corporate Directors. He has also served as Chairman of the Canadian Chamber of Commerce; Chairman of the International Chamber of Commerce – Canada; and Member of the Canada/U.S. Trade Committee. Dr. Wilson draws on this experience to ensure Flora Growth adheres to effective corporate governance practices.
Luis Merchan is the company’s President and CEO. He is a proven executive with over a decade of experience in enterprise sales management, corporate strategy, merchandising and expense management, and customer experience. Mr. Merchan previously served as Macy’s Inc.’s Vice President of Workforce Strategy and Operations, where he managed the enterprise’s multi-billion-dollar P&L expense line for the entire 540 store portfolio. Throughout his tenure at Macy’s, he led various sales and marketing initiatives, including the B2B corporate sales team that was responsible for $160 million in annual revenue. Mr. Merchan obtained his Bachelor of Industrial Engineering from Pontifical Xaverian University in Bogota, Colombia, and his MBA from McNeese State University. He also holds a Graduate Certificate in Marketing Management from Harvard.
Juan Manuel Galan is a Strategic Advisor to the Flora Growth management team. Mr. Galan currently serves as a senior consultant to The World Bank. He is a politician and former senator of Colombia, serving three terms from 2006 to 2018 as a member of the Colombian Liberal Party. He is also a former professor at the University of Rosario and holds more than 20 years of journalistic, academic, governmental and parliamentary experience. During his time as a senator, Mr. Galan was a key leader, with 29 bills and 27 debates on political control, and 17 laws to his name. The most relevant of those laws was authoring the medical cannabis law that resulted in the legalization of medical cannabis in Colombia.
Stan Bharti is a Director of Flora Growth. Mr. Bharti currently serves as Executive Chairman of Forbes & Manhattan. He has more than 30 years of professional experience in business, finance, markets, operations and more, with a focus on the resource and technology sectors. To date, Mr. Bharti has amassed over $3 billion worth of investment capital for the companies with which he has worked and their shareholders. He is a Professional Mining Engineer and holds a master’s degree in engineering from Moscow, Russia, and University of London, England.
Javier Franco is the company’s VP of Agriculture. Mr. Franco is a master horticulturist with more than 25 years of experience in the design, implementation, and management of cultivation and propagation facilities of more than 30 species of cut flowers in Latin America. He completed his agricultural studies at Zamorano University in Honduras and later at an International Exchange Program at Ohio State University. Mr. Franco has directed technical, commercial, and research groups in the cut flower, fruit and vegetable markets in Latin America and has participated in the commercial development of new technologies applied in agribusiness. He has also led the agri-management of organic crops and certifications of Good Agricultural Practices.
Foresight Autonomous Holdings Ltd. (NASDAQ: FRSX) (TASE: FRSX)
Foresight Autonomous Holdings Ltd. (NASDAQ: FRSX) (TASE: FRSX), founded in 2015 and headquartered in Israel, is a technological innovator in automotive vision systems and driver assistance technology. Through its wholly owned subsidiary, Foresight Automotive Ltd., Foresight is engaged in the design, development and commercialization of stereo/quad-camera vision systems and V2X cellular-based solutions for the automotive industry based on 3D video analysis, advanced algorithms for image processing and sensor fusion. The company’s powerful and patented stereoscopic technology is derived from field-proven technology that has been deployed throughout the world for almost two decades.
Foresight’s innovative autonomous driving solutions are based on mature, proprietary stereoscopic image technology that uses two synchronized cameras to mimic human depth perception and produce a three-dimensional image. This 3D image can anticipate possible collisions with other vehicles, cyclists, pedestrians and other obstacles. The technology provides highly accurate real-time alerts about the vehicle’s surroundings while in motion. The systems are designed to improve driving safety by enabling highly accurate and reliable threat detection while ensuring the lowest rates of false alerts.
The company’s patents provide IP protection for its robust and proven proprietary stereoscopic technology, which was developed using the security technology of Foresight’s major shareholder, Magna B.S.P.
Foresight has developed three main products:
- QuadSight™. This breakthrough detection system sets the bar for autonomous vehicle vision. It features nearly 100 percent obstacle detection with almost zero false alerts and operates optimally under all weather and lighting conditions, including darkness, rain, fog, haze and glare. QuadSight™ is the first quad-camera multi-spectral vision solution of its kind, driven by advanced and proven image processing algorithms. The system consists of two sets of stereoscopic infra-red and visible-light cameras that enable highly accurate and reliable obstacle detection for seamless 24/7 vision.
- Eyes-On™. This solution uses advanced algorithms for accurate depth analysis and obstacle detection to provide a unique stereo vision Advanced Driver Assistance System (ADAS). It can detect all potential obstacles regardless of shape, form or material, including other vehicles, cyclists, pedestrians and animals. It has an accuracy and reliability of almost 100 percent and near zero false alerts.
- Eye-Net™. This is a cellular-based accident prevention solution that is designed to provide real-time pre-collision alerts to vehicles and pedestrians. This proprietary system is deployed on smartphones and cloud-based servers operating on existing cellular networks, and it eliminates the need for additional designated hardware. Eye-Net™ is designed to provide a complementary layer of protection to advanced driver assistance systems and extends this protection to road users who are not in direct line of sight. It is optimally designed for both urban environments and high-speed scenarios to provide protection for the most vulnerable road users. On March 28, 2018, Foresight announced that it had completed a successful feasibility study of its Eye-Net™ accident prevention solution involving 120 users of Android and iOS cell phones located across Israel.
In 2017, Foresight sought more opportunities within the international market. The Company signed pilot agreements with three leading car manufacturers in China and completed pilot projects meeting all pre-defined requirements and criteria. In addition, FRSX completed a pilot project with Uniti Sweden.
Studies by the Insurance Institute for Highway Safety continue to emphasize the dramatic reduction in accidents and injury-related crashes reported when vehicles are equipped with collision avoidance systems. A recent study by the Institute states that the rate of single-vehicle, sideswipe and head-on crashes was 11 percent lower in vehicles with the warning systems. More importantly, the study shows collision avoidance technology cut the rates of injury crashes of the same type by 21 percent.
Foresight Autonomous Holdings, Inc. also holds a 32 percent interest in RailVision, a company that develops advanced systems for railway safety and maintenance. RailVision has successfully completed 13 tests in Israel, Germany, Italy and Switzerland in addition to a real-time system test with a European railway operator. Over the course of 2017, RailVision successfully completed rounds of financing totaling $5.8 million and started the process of licensing the system according to European standards.
Haim Siboni is the founder of Foresight and has served as the company’s chief executive officer and director since 2015. Siboni, a passionate entrepreneur, has an extensive background in the marketing and business management sectors in the fields of electronics, video, TV, multimedia, computerized systems, line and wireless telecommunication, design and development of systems and devices, including electro-optic radar systems. He is the founder and CEO of Magna B.S.P., Foresight’s major shareholder and a leading innovator in the field of homeland security surveillance solutions.
Freight Technologies Inc. (NASDAQ: FRGT)
Freight Technologies Inc. (NASDAQ: FRGT) (“Fr8Tech”) is a technology company developing solutions to optimize and automate the supply chain process, providing a platform for B2B cross-border shipping in the NAFTA region. The company’s mission is to revolutionize cross-border shipping by providing carriers with increased growth opportunities and shippers with flexibility, visibility and simplicity for the once-complex process of international over-the-road shipping.
Freight Technologies, formerly known as Hudson Capital Inc., assumed its current name and ticker symbol on May 27, 2022. Its primary operating subsidiary and its marketplace are known as Fr8App, and it conducts operations throughout North America under the names of Fr8App and/or Freight App. The company is headquartered in Houston, Texas, with multiple locations across the U.S. and Mexico.
The Fr8Tech Solutions Suite
Fr8Tech leverages artificial intelligence to provide cloud-based platforms aimed at automating the over-the-road transportation process, effectively reducing human touch points and expediting load booking times. The company’s suite of solutions includes:
- Fr8app – A B2B marketplace powered by AI and Machine Learning offering a real-time broker portal to connect shippers with qualified carriers
- Fr8Radar – A tracking solution providing shippers and carriers real-time locational data via Fr8app’s mobile solution or through integration with third-party GPS alternatives
- Fr8TMS – A transportation management system designed to help shippers manage their freight and all of the documents involved in shipping transactions, including invoices, customs documents, confirmation rates and proof of deliveries
- Fr8FMS – A fleet management system allowing transportation companies to better manage their fleets, reduce operational costs and provide better service to their customers
- Fr8Data – A data solution offering real-time dashboards and reports to shippers and carriers in an effort to increase visibility and control while supporting better business decisions
- Fr8Fleet – A platform that provides private fleet management, enabling large corporate shippers to purchase dedicated capacity secured by Fr8app in exchange for a fixed fee
Commitment to the Environment
Through its core focus on technology, Fr8Tech seeks to reduce the carbon footprint of the logistics industry. Its solutions aim to minimize empty miles for transportation firms and reduce overall paper consumption.
Fr8University
Fr8University is an educational program offering classroom and on-the-job training for Fr8Tech team members. Through the program, employees learn in-depth business fundamentals and applications along the truckload freight industry value chain.
Led by corporate educator Mario Mena, Fr8University is designed as an investment in the company’s human capital, providing an opportunity to communicate Fr8Tech’s corporate culture while accelerating operational growth.
Market Outlook
Fr8Tech’s established foothold in Mexico is key to its current efforts to promote sustainable growth in the cross-border shipping industry. Ongoing disruption in U.S.-Chinese trade relations have strengthened Mexico’s status as the largest trading partner of the U.S., with cross-border annual freight spending estimated at $385 billion according to data from the U.S. Department of Transportation. Annual domestic shipping in Mexico is estimated at $34 billion, while annual domestic shipping in the U.S. is estimated to total $732 billion.
Despite the size of this industry, fragmentation and inefficiencies prevail in the space. Thousands of legacy brokers, tens of thousands of shippers and hundreds of thousands of carriers still rely on outdated systems to arrange transport, spending hours on the phone negotiating pricing, waiting days to find trucks and drivers, preparing and printing forms, and operating without tracking or visibility. Add in cross-border complexity relating to customs and additional paperwork, and you have an industry ripe for technological disruption.
Fr8Tech’s recent revenue growth trends have highlighted the company’s efforts to capitalize on this opportunity. In 2021, Fr8Tech achieved revenues of $21.5 million, marking a year-over-year increase of 134%. The company issued revenue guidance for fiscal 2022 of $40 million in a February 9, 2022, press release, which would account for a further 86% year-over-year increase.
Management Team
Javier Selgas is CEO and a Director of Freight Technologies Inc. and Freight App Inc. He brings to the company over 15 years of experience developing technology and digital marketing strategies, including serving as Country Manager for Osigu, Spain, and as head of AJEgroup’s IT division for the Asia-Pacific region. Prior to joining Fr8Tech, Mr. Selgas founded digital marketing agency Lanzadera Online. He has also served as an IT consultant to major corporations, including Endesa and Ibermatica.
Mike Flinker is President of Fr8Tech. He has over four decades of experience in the transportation industry, with 30+ years focused on cross-border logistics. Prior to joining Fr8Tech, Mr. Flinker founded FLS Transportation, the largest cross-border logistics company in Canada. He also previously held positions with Clarke Transport Inc., Canadian Pacific and Reimer Express Inc. (a division of Roadway Express).
Paul Freudenthaler is the company’s CFO and Secretary to the company Board. He has over 30 years of financial expertise, having previously served as CFO for several leading companies across multiple countries, including Macquarie in Mexico, Old Mutual in Latin America and Ascentium Capital in the U.S. Mr. Freudenthaler’s experience include leadership roles from which he guided IPOs and M&A transactions.
Luisa Lopez is COO of Fr8Tech. She brings to the company 25+ years of management experience in logistics, supply chain, operations and customer service. Ms. Lopez previously served as a Director of Landstar, where she was responsible for commercial and client development strategies in the Mexican market. Additionally, she managed more than 2,000 transport units specialized in staff and school mobility while with Traxion in Mexico.
Friendable Inc. (FDBL)
Friendable Inc. (FDBL) is a mobile technology and marketing company focused on connecting and engaging users through its proprietary mobile and desktop applications. Launched July 24, 2020, the company’s flagship offering is designed to help artists engage with their fans around the world and earn revenue while doing so. The livestreaming platform supports artists at all levels, providing exclusive artist content ‘Channels’, LIVE event streaming, promotional support, fan subscriptions and custom merchandise designs, all of which serve as revenue streams for each artist.
With Fan Pass, artists can offer exclusive content channels to their fans, who can use their smartphones to gain access to their favorite artists, as well as an all-access pass to all artists on the platform. Additionally, the Fan Pass team will deploy social broadcasters to capture exclusive VIP experiences, interviews and behind-the-scenes content featuring their favorite artists – all available to fan subscribers on a free trial basis. Subscriptions are billed monthly at $3.99, or about the cost of downloading a couple of songs, and VIP experiences are available at a fraction of the cost of traditional face-to-face meetups.
Friendable Inc. was founded by Robert A. Rositano Jr. and Dean Rositano, two brothers with over 27 years of experience working together on technology-related ventures.
The Fan Pass Mobile & Desktop App
Friendable Inc. launched its Fan Pass platform as a solution for artists and their fans as the COVID-19 pandemic and the associated shutdown have continued to severely hamstring the entertainment industry as a whole. Through Fan Pass, the company aims to reach artists at all levels looking to alter their touring schedules to include ‘Virtual Touring’, new revenue sources and innovative fan engagement opportunities that are expected to become permanent fixtures of artists’ touring routines moving forward.
Fan Pass creates an ecosystem that embraces fans of all kinds, feeding diehard followers and developing lasting connections with more casual supporters. Through the app, qualified artists are provided with a custom designed, exclusive ’Fan Pass Channel’ where they can invite fans and social followers from anywhere around the world to join in chats and live events – allowing fans to experience all there is to see of an artist in one place. Artists earn revenue from monthly fan subscribers, merchandise sales, tickets sold for virtual streaming events and generally from all content views or impressions on their channels. All content views and sales of every kind are reported to each artist through their dashboards, including real-time payout and earnings information.
Fan Pass’ exclusive ‘All Access VIP’ option provides fans with access to content, such as:
- Live performances or online concerts
- Backstage meetups before, during or after events
- Livestreams of studio sessions
- Behind-the-scenes footage of music video and photo shoots
- Special interviews and one-on-one videos
- Streams highlighting the artists’ daily lives
The Fan Pass platform is extremely intuitive, bringing each artist through a streamlined onboarding process, including building out artist ‘Channels’, scheduling LIVE events and designing special edition merchandise to be offered solely through exclusive Fan Pass merchandise stores.
“With the global pandemic disrupting the entertainment industry in such a profound way, artists have had to look to digital distribution and live virtual performances in order to maintain any earning opportunities. Fan Pass and our team are determined to provide solutions and support to all artists, their fans and the industry in general. We are excited about the opportunity we have to shape the future of virtual entertainment, revenue generation and artist/fan engagement,” Robert A. Rositano Jr., CEO of Friendable Inc., stated in a news release.
Market Opportunity
Artists rely heavily on revenue streams that are not often seen by those without intimate industry knowledge. When it comes to traditional performances, the sale of VIP/backstage or meet & greet passes to boost revenue can often become the majority of the artist’s annual tour revenue. Data provided by one of the company’s original entertainment partners, The Kluger Agency (TKA), suggests that as much as 18-23% of artists’ annual tour revenue has historically been derived from these VIP experiences.
The World Economic Forum reports that, in 2020, the six-month-plus disappearance of live music concerts is estimated to have cost “the industry more than $10 billion in sponsorships,” and individual artists are feeling the loss the most. Fan Pass is helping to bridge this gap, providing more affordable virtual VIP experiences that can be offered simultaneously to fans around the world.
While it’s free for artists to join, Fan Pass leverages a monthly subscription model paid by fans to generate revenues. These revenues are shared with all channel artists. In exchange for its platform features, live streaming tools, bandwidth, processing and handling, Fan Pass earns platform fees on each separately ticketed event, as well as splits with each artist on subscriber fees and merchandise designed and sold on the platform.
The U.S. video streaming industry is expected to hit $7.08 billion in value in 2021, with an estimated 100 million internet users watching online video content every day, according to data from Livestream.com. The same report suggests that 45% of live video audiences would pay for exclusive, on-demand video from a favorite team, speaker or performer. Through Fan Pass, Friendable Inc. is uniquely positioned to capitalize on this opportunity.
Friendable App
The company’s second application, Friendable, is an all-inclusive platform where users can meet, chat and date. The app has exceeded 1.5 million total downloads, with over 900,000 historical registered users and more than 580,000 historical user profiles.
Friendable Inc.’s Next Phase of Growth
To facilitate its next phase of growth, Friendable Inc. is seeking an additional $1 million in equity investment, with a follow-on funding that meets or exceeds $5 million. The company intends to utilize its relationships to secure the lowest cost of capital available, as these funds will drive technology advancements, increase head count, fund marketing initiatives and secure additional celebrity talent aimed at bringing larger fan audiences to each released event. These initiatives will assist in building recurring monthly (fan) subscribers, effectively generating recurring monthly revenue for each artist, as well. The next phase of growth is expected to play a key role in accelerating the company’s download and conversion of data for subscription revenue and merchandise sales.
The company’s primary goal is to establish Fan Pass as a premier brand and mobile platform dedicated to connecting and engaging users around the world. In support of this goal, it has entered into a partnership with Brightcove targeting OTT platform expansion, including leaders such as iOS, Android, Apple TV, Android TV, Roku and WWW.
In the highly competitive video streaming market, Friendable Inc. has tapped into an unmet demand from today’s ever-present ‘omni-users’ for constant contact with celebrities and influencers. Via Fan Pass, the company offers investors an opportunity to gain a stake in an organization catering to this new breed of omni-users and their influencers.
The application’s potential is clearly illustrated by the interest it has generated in recent weeks. From September 4 to October 12, the Fan Pass platform added 246 new artists, accounting for a 410 percent increase in just six weeks.
“We are extremely encouraged by the ongoing swell of interest as the value of our Fan Pass platform continues to resonate in the artist community,” Friendable CEO Robert A. Rositano Jr. stated in a news release. “We believe the live streaming functionality, our full-circle offering and diverse revenue opportunities the platform offers will continue to drive exponential growth as management remains focused on building long-term shareholder value.”
Management Team
Robert A. Rositano Jr. is the co-founder and CEO of Friendable Inc. He oversees the daily management and operational duties of all areas of the business. He has over 20 years of experience as a serial entrepreneur, bringing in over $60 million in liquidity events for the companies he has created or managed. Before starting Friendable Inc. with his brother, Rositano was a founding member of the internet’s first IPO, Netcom Online Communications Inc. It was sold to ICG, then to EarthLink in 1995. He has been a co-founder of several successful ventures, including Simply Internet Inc., Nettaxi.com and America’s Biggest Inc., among others. He also authored one of the first web directories for MacMillan Publishers.
Dean Rositano is the co-founder and Chief Technology Officer of Friendable Inc. He handles the day-to-day operations and guides the technical direction of the company. He has over 15 years of executive management, financial management, high technology operations and internet architecture experience. Before co-founding Friendable Inc., Rositano co-founded several other companies, including Checkmate Mobile Inc. and Latitude Venture Partners LLC, among others.
FuelPositive Corp. (TSX.V: NHHH) (OTC: NHHHF)
FuelPositive Corp. (TSX.V: NHHH) (OTC: NHHHF) is a growth stage company focused on licensing, partnership and acquisition opportunities building upon various technological achievements. The company is committed to providing commercially viable and sustainable clean energy solutions, including carbon-free ammonia (NH3), for use across a broad spectrum of industries and applications.
FuelPositive is headquartered in Toronto, Canada.
Hydrogen Economy Problems and FuelPositive’s Carbon-Free Technology
The hydrogen economy is currently facing many challenges. Traditional NH3 manufacturing exists on a massive scale, but centralized facilities result in some of the world’s most concentrated CO2 emissions. In total, an estimated 200 million metric tonnes of NH3 are consumed each year, with greater than 80% utilized by the agricultural sector. NH3 is also being positioned as a viable alternative to fossil fuels.
FuelPositive’s flagship carbon-free ammonia technology provides an innovative solution to these environmental concerns. Developed by Dr. Ibrahim Dincer and his team, the company’s platform allows for the in-situ production of NH3 in an entirely sustainable manner, using only water, air and sustainable electricity.
The production of hydrogen is energy intensive, but it is just one variable hindering the growth of the hydrogen economy. Other hurdles include:
- Storage – The storage of hydrogen by compression or liquification are both cost prohibitive and unsustainable.
- Distribution – The distribution network for effective hydrogen deployment has yet to be developed, as the extreme high-pressure distribution requirements to transport hydrogen would result in enormous infrastructure costs.
- End Use – R&D on the transportation-related end use applications for hydrogen is in its infancy, but almost any vehicle on the road today can be easily converted to run on NH3 at a considerably lower cost per mile traveled when compared to traditional fossil fuels.
A key benefit of FuelPositive’s patent-pending, first-of-its-kind carbon-free NH3 technology is its flexibility. The process allows for small, medium or large-scale production of NH3 on location, minimizing or even eliminating the challenges and volatility associated with storage and transportation to end use. As such, with an appropriately sized FuelPositive system and access to renewable energy, the end use applications for the company’s platform are nearly infinite.
Manufacturing Partnership
On May 19, 2021, FuelPositive announced its selection of National Compressed Air Canada Ltd. (“NCA”) to undertake manufacturing of the company’s Phase 2 hydrogen-ammonia synthesizer commercial prototype systems for carbon-free ammonia production.
In a news release detailing the partnership, FuelPositive CEO Ian Clifford noted, “This critical milestone for FuelPositive will confirm the broad application potential for our technology and is the backbone of our Carbon-Free Hydrogen-NH3 offering. Partnering with the knowledgeable and experienced team at NCA on this commercialization project will bring our development-stage program to life.”
Global Ammonia Market Outlook
The global ammonia market was valued at $52.71 billion in 2017 and is forecast to reach $81.42 billion by 2025, growing at a CAGR of 5.59%, according to data from Fior Markets (https://ibn.fm/1OfOB).
The agricultural industry consumes more than 80% of global NH3. Smaller percentages can be attributed to the waste, water treatment, refrigerants, antiseptic, textile, mining and pharmaceutical industries.
One of the most polluting industries on the planet consists of conventional agribusinesses. These polluters are responsible for more greenhouse emissions per year than transportation. This is where FuelPositive’s technology is expected to be extremely beneficial.
Management Team
Ian Clifford is Director, CEO and Founder of FuelPositive Corp. He has over 25 years of experience in the fields of technology and marketing and has successfully led the company to global brand recognition through its unique energy solutions. Since 2006, Mr. Clifford has raised over $50 million in equity financing for FuelPositive. He also co-founded digIT Interactive, a full-service internet marketing company serving Fortune 500 clients, which he sold at the peak of the market in 2000.
Greg Gooch serves as a Director and President of FuelPositive. His multifaceted career in the electronics and finance industries has positioned him as a key advisor and funding partner to start-ups and new technology companies for over 40 years. Mr. Gooch has been involved with FuelPositive since its early days and has remained a significant supporter and consultant to the company over the years. He has a bachelor’s from McGill University and an MBA from the University of Western Ontario.
Dr. Ibrahim Dincer is a scientific advisor to FuelPositive and is recognized as a pioneer and international leader in the area of sustainable energy technologies. Along with his team, Dr. Dincer invented the modular carbon-free ammonia (NH3) production technology that FuelPositive is commercializing. His area of specialty covers various topics including ammonia, hydrogen energy and fuel cells; renewable energy systems; energy storage systems and applications; carbon capturing technologies, and integrated and hybrid energy systems He is currently managing an exemplary team of researchers in this commercialization project.
Marek Warunkiewicz is the company’s Communications & Branding Specialist. He brings more than 40 years of entrepreneurial expertise to the FuelPositive team, having held marketing, branding, advertising, project management and graphic design positions with various companies. Mr. Warunkiewicz has successfully created business-to-business marketing and advertising campaigns for a diverse group of clients ranging from high-tech to agriculture. He co-founded digIT Interactive and ZENN Motor Company alongside Ian Clifford.
Luna Clifford is the Director of Communications for FuelPositive. She has over 10 years of experience as a business owner and advisor, helping build and operate several successful start-up enterprises while managing complex stakeholder relationships. Ms. Clifford excels in strategic planning and team building, and she has completed extensive studies in the fields of communications and health care.
GeoSolar Technologies Inc.

GeoSolar Technologies Inc. (“GST”) is a Colorado-based climate technology company and the creator of the Smart Green Home® system for newly built and existing residences and commercial buildings. The company is focused on revolutionizing the way we heat, cool and power homes with 100% natural energy sources. Its patent-pending integrated system harnesses energy from the earth and sun to power and purify homes and automobiles without the use of fossil fuels.
In a GST home, the sun’s energy is captured on the roof to generate all of the electricity required. Additionally, the consistent climate of the earth is used to keep the home at a perfect temperature year-round, and the company’s proprietary air purifying unit ensures that the air inside the home is safe and healthy.
GST’s home technology has been installed in multiple test homes in Colorado and achieved exceptional results, including some of the most impressive energy efficiency ratings (HERS) in the industry.
GeoSolar Technologies is currently accepting investment as part of a Regulation A+ offering. Everyone* can invest now for as little as $300. For more information, visit the company’s profile on Manhattan Street Capital and review its Offering Circular.
GeoSolar Technologies Inc. (“GST”) has been qualified by the U.S. Securities and Exchange Commission (SEC) to conduct a Regulation A+ capital raise. GST is already a publicly traded company who makes quarterly and annual filings with the SEC and is subject to quarterly PCAOB audits. This is the first time shares of GeoSolar Technologies are being made available for public purchase. Upon completion of this Regulation A+ offering, the company intends to seek a listing of its stock.
The Decarbonization Movement
 Soaring and unstable energy/fuel costs continue to highlight the importance of rethinking the traditional approach to powering homes, from top to bottom. While most everyone is well aware of the remarkable, multi-trillion-dollar opportunity the electric vehicle transformation offers to investors (in addition to the benefits to the climate problem), few recognize that the all-electric home market is as large as electric vehicles and equally important to reducing carbon emissions.
Soaring and unstable energy/fuel costs continue to highlight the importance of rethinking the traditional approach to powering homes, from top to bottom. While most everyone is well aware of the remarkable, multi-trillion-dollar opportunity the electric vehicle transformation offers to investors (in addition to the benefits to the climate problem), few recognize that the all-electric home market is as large as electric vehicles and equally important to reducing carbon emissions.
U.S. energy expenditures clocked in at $3,891 per person in 2018, leading to estimated spending of $1.3 trillion on energy that year alone. Despite this, fewer than 3% of U.S. homes are currently powered by solar. This number is poised to increase exponentially as both new and existing residences transition to zero carbon models.
GST estimates that if all the homes in America were powered by its technology, carbon pollution could be reduced by an estimated 1.9 trillion pounds per year, greatly reducing the negative impacts on our climate.
GeoSolarPlus®
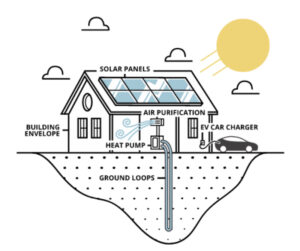 The GeoSolarPlus (“GSP”) system combines solar power, geothermal ground-sourced energy and other clean energy technologies into one fully integrated system.
The GeoSolarPlus (“GSP”) system combines solar power, geothermal ground-sourced energy and other clean energy technologies into one fully integrated system.
Key benefits of the GSP system include:
- Making a real planet-changing difference in reducing air pollution
- Eliminating or significantly reducing homeowners’ future utility bills
- Enjoying lifetime energy independence and protection from price escalation and energy shortages
- Eliminating greenhouse gas emissions from operation of home and daily life
- Increasing home value
- An integrated design for seamless operation of renewable energy systems
- Maintaining a significantly healthier living environment
- Leveraging existing renewable energy tax credits and electrification incentives
- Creating stable jobs capable of supporting families in the decarbonized future
Click here to learn more about how GeoSolarPlus works.
Management Team
The GST leadership and management team includes some of the world’s most experienced and respected leaders in the fields of decarbonization and sustainable homes.
Stone Douglass is the Chairman and CEO of GST. He is a seasoned, 30-year public company executive and former Chairman and CEO of the Piper Aircraft Company.
Brent Mosbarger is the company’s Co-Founder and leads its commercial operations. He is a highly respected solar engineer whose experience includes roles with Chevron Energy’s green operations and serving as project manager and executive for a $400 million solar/geothermal innovation project.
Peter Romenesko is a Senior Strategic Advisor with GST. He brings to the company considerable experience as an engineer and large-scale project manager for Johnson Controls and Siemens.
Dr. Norbert Klebl is the company’s Co-Founder and Development Director. Recognized as one of the world’s leading experts in the field of zero-carbon innovation, he is a former McKinsey partner of 16 years with an MBA from Columbia.
Dar-Lon Chang is GST’s Director of New Product Development. Prior to joining GST, he had a 16-year career with ExxonMobil Energy Research. He received his PhD in engineering from the University of Illinois.
* Must be over 18, certain states are not currently available and will be added soon.
Golden Matrix Group Inc. (NASDAQ: GMGI)
Golden Matrix Group Inc. (NASDAQ: GMGI), based in Las Vegas, Nevada, is an established gaming technology company that develops and owns online gaming IP and builds turnkey online casino solutions for gaming operators as well as configurable and scalable white-label gaming platforms for international customers, located primarily in the Asia-Pacific region. GMGI’s gaming IP includes tools for marketing, acquisition, retention and monetization of users. The company’s platform can be accessed through both desktop and mobile applications.
GMGI’s sophisticated software automatically declines any gaming or redemption requests from within the United States, in strict compliance with U.S. law.
Golden Matrix, through a subsidiary, also runs a pay-to-enter prize competition in the United Kingdom and Ireland.
The company’s shares began trading on the Nasdaq under the symbol ‘GMGI’ on March 17, 2022. Golden Matrix shares were previously traded on the OTCQX Best Market.
For the quarter ended January 31, 2022, the company reported revenue of $8.88 million, an increase of 355% over the same quarter one year earlier. Net income for the three-month period was $349,379, up from $52,158 a year earlier. It was the company’s 14th consecutive profitable quarter.
In December 2021, Golden Matrix announced it had entered into a purchase agreement to acquire a controlling ownership interest in UK-based RKingsCompetitions Ltd., one of Ireland’s and the United Kingdom’s leading independent online competition companies. RKings presents customers with paid and free entry routes to competitions that offer a range of prizes, including residential properties, luxury and exotic motor vehicles, holiday packages, technology packages and cash. The competitions are currently open only to residents of Ireland and the United Kingdom. Golden Matrix acquired an 80% ownership interest in RKings for cash and stock. The company also secured an option to purchase the remaining 20 percent interest of RKings, subject to certain requirements.
In March 2022, Golden Matrix announced it had applied for a Mexican gaming permit and, once approved, expects to offer online gaming in Mexico as well as roll out the RKings tournament business globally.
Technology
Golden Matrix Group develops fully operational online casino turnkey solutions as well as highly modular, configurable and scalable gaming platforms for its international customers in an effort to promote user acquisition, engagement, retention and monetization. The provided white label gaming platform is unparalleled in both mobile and desktop website deployment, proving compatible throughout all major operating systems and web browsers. In addition, the platform enhances the client’s ability to cater to various gaming scenarios including but not limited to transaction management and a range of loyalty and reward programs. Moreover, user engagement is optimized through the ability to accommodate both free and paid games.
The company’s GM-X System (and recently its next generation GM-Ag System) is considered the industry standard, granting access to over 10,000 games from more than 25 game providers. Through the GM-X System, Golden Matrix offers the industry’s most extensive game portfolio. The company’s gaming partners dominate the global online gaming market to deliver innovative games and premium brand titles. The GM-X System offers payment gateways that integrate with third party platforms or digital wallets. It supports all major currencies and offers multiple language options. The system’s data analytics provide the operator with a 360-degree view of the gaming platform’s performance.
GMGI currently supports over 500 unique casino brands and over 6 million players.
Market Outlook
Online gaming and sports betting sites and apps are increasingly taking market share from traditional location-based casinos. Widespread internet service availability and increasing use of mobile phones for playing online games from homes and public places is driving the market, according to a report from Grand View Research. In addition, factors such as easy access to online gambling, legalization and cultural approval, corporate sponsorships, and celebrity endorsements are also contributing to market growth. The growing availability of cost-effective mobile applications across the globe is further expected to fuel market growth.
This trend is only expected to accelerate as millennials reach their peak earning years and Gen Z youth begin to complete their education and move into careers. These generations are completely comfortable with online recreation, and with using technology like digital wallets and digital gameplay that underpins online gaming.
The global online gambling market was valued at $53.7 billion in 2019 and is expected to grow at a CAGR of 11.5% from 2020 to 2027 to reach a value of $127.3 billion, according to Grand View Research, with much of the growth expected from the U.S. and Asia. Even Europe, the most mature gaming market, is expected to grow at a rate of 20-25% year-over-year.
Management Team
Brian Goodman is CEO of Golden Matrix Group. He has more than 20 years of diverse senior management experience and business development roles within the technology and internet gaming industries. He has a tertiary science qualification as well as a marketing and sales background. His previous roles have been entrepreneurial and include CEO and senior management positions in smaller organizations, which he founded or in which he held equity, as well as multinational organizations.
Cathy Feng is COO at Golden Matrix. She is a co-founder of GMGI and holds a Master of Commerce degree. She has 10 years of experience as a financial officer in the technology and internet gaming industries. In past management positions, she interpreted, analyzed and presented financial and operation information to facilitate business decisions, grow companies and resolve complex problems. In addition, she has skills in marketing, business development, leadership and strategic planning.
Omar Jimenez is CFO and Chief Compliance Officer at GMGI. Prior to joining the company, he was CFO and COO of Alfadan Inc., a supplier of marine outboard engines. He has held senior financial management and operational positions at public and private companies including NextPlay Technologies, American Leisure Holdings, US Installation Group and Onyx Group. He holds various accounting professional certifications, including CPA and CPCU, and degrees in finance, accounting and business.
Henry Zhang is Chief Technology Officer at Golden Matrix. He oversees all aspects of development, integration and deployment of GMGI’s technology systems. He plays a key role in evolving GMGI’s technology business to lead and shape the industry. He is responsible for developing and scaling new businesses, including online gaming, eSport and P2P Systems. He was instrumental in launching the GM-X system and has been with the company for more than six years.
Golden Triangle Ventures Inc. (OTC: GTVH)
Golden Triangle Ventures Inc. (OTC: GTVH) is a multifaceted consulting company pursuing ventures in the health, entertainment and technology industries, with many additional projects being developed that provide synergistic values to these divisions. The company aims to purchase, acquire and/or joint venture with established entities that management can help assist and develop into unique opportunities.
Additionally, GTVH provides a professional corporate representation service to different companies in these sectors while consulting on a variety of business development objectives. The goods and services represented are driven by innovators who have passion and commitment to these marketplaces.
The company plans to utilize relationships and create a platform for new and existing businesses to strengthen their products and/or services. The three points of the Golden Triangle exclusively represent these three sectors in which the company aims to do business.
Health Division – Global Health Services
Global Health Services is a wholly owned subsidiary of Golden Triangle Ventures (operating under its Health Division). Dedicated to the promotion of well-being and natural wellness, the company currently does business in the industrial hemp/CBD industry. Additionally, the company has a vision to promote, market and generate sales for a myriad of products and services which include a full retail line of high-end, all-natural health, wellness and beauty products.
To help achieve this vision, Global Health Services is in the process of further developing an extensive online portal that will support the multiple verticals under the company and provide a one-stop-shop for all of the company’s products and services. Moreover, to support overarching business goals, senior management tirelessly works on acquiring and building an array of profitable assets and projects.
Entertainment Division – Lavish Entertainment
Lavish Entertainment (EpicRaves) is a wholly owned subsidiary of Golden Triangle Ventures under its Entertainment Division. Operating out of Las Vegas, Nevada, the company started doing business in 2017 and was established with a vision of becoming a nationally recognized concert production company. The company currently has more than 30,000 national followers and nearly 100 team members who have helped the company successfully organize some of the most exciting Electronic Dance Music concerts in Las Vegas.
Lavish Entertainment is currently doing business as EpicRaves, which will eventually become a wholly owned subsidiary of Lavish Entertainment as the company expands its business into a variety of other forms of entertainment. The company is currently building a unique virtual reality platform to help expand on its live events, and it is working to acquire a 68,000 sq. ft. event center with a vision to develop one of the most advanced event centers in the world.
Technology Division – HyFrontier Technology
HyFrontier Technologies is a wholly owned subsidiary of Golden Triangle Ventures under its Technology Division. The company owns a patent-pending process and device technology called HyGrO, which is a molecular hydrogen and oxygen delivery system for agriculture. Golden Triangle Ventures is assisting the company in commercializing the HyGrO unit for farm and home use in markets across the globe. HyFrontier Technologies has a mission to improve global crop production efficiency by producing hydrogen and oxygen directly in the water stream.
This technology can be used on any species of plant life in nearly any grow medium. Additionally, the system can be retrofitted to wellheads for large-scale agricultural projects, indoor grow operations and small farms or utilized for a multitude of residential home and garden applications. In-house testing has shown evidence that hydrogen is capable of increasing crop yields by up to 25% and, in many circumstances, a much higher amount. Larger root systems and better overall plant health were also observed by watering plants with the HyGrO unit. Universities and multiple third-party testing facilities are currently working to validate the HyGrO technology, and all preliminary results are extremely positive.
To push the development and commercialization of the technology, management is now in the process of moving the company headquarters from Colorado to Florida, which will transition its operations into a 7,800 sq. ft. state-of-the-art manufacturing facility. The company recently executed a three-year lease with an option to purchase the entire 24,000 sq. ft. building, which will help the business in achieving its ultimate goal of commercializing this technology to the world.
Food & Wine Division – Napa Wine Brands
Napa Wine Brands is a wholly owned subsidiary of Golden Triangle Ventures which is a synergistic business with a mission of providing a world-class portfolio of unique brands birthed from Napa Valley and Sonoma Valley in the heart of California’s Wine Country.
The company has a commitment to manufacture and distribute specialty wines, foods and unique items while tapping into an array of hidden markets in the food and beverage industry. With extensive resources and award-winning products, Napa Wine Brands aims to develop some of the most desirable products in today’s market. Originated by some of the most profound experts in Napa Valley, the company’s vision is to broaden the horizon of a traditional food and wine company by creating a platform different than anything seen in the Northern Hemisphere.
Napa Wine Brands has an array of fully developed products and services that provide value to the other divisions under Golden Triangle Ventures. The company is now preparing the launch of several brands, products and services that are market-ready and will immediately turn into cash-positive businesses. Golden Triangle Ventures will provide a full support system and assist management of Napa Wine Brands in growing this company into another fun, exciting and profitable division of Golden Triangle Ventures.
Recent Updates
- On May 26, 2021, Golden Triangle announced its acquisition of The Lodge Winery & Olive Oil Co. under the company’s Napa Wine Brands subsidiary. The Lodge Winery & Olive Oil Co. is an established wine brand that produces award-winning wines, olive oils and wine vinegars. “Our marketing team is now ready to launch an in-depth program focused on driving our products into big box stores, smaller retail outlets, online platforms and many other avenues,” Steffan Dalsgaard, CEO of Golden Triangle, stated in a news release announcing the acquisition. “We are working directly with [Napa Wine Brands CEO] Arron [Johnson] and his team to grow their bulk inventory and launch all of these products for the world to enjoy.”
- On May 20, 2021, Golden Triangle announced its entry into a letter of intent to acquire Sonder Fulfillment LLC, a leader in the industrial hemp and CBD space that is dedicated to driving forward the most powerful and efficacious cannabinoid products in the world. “Over the past two years, our operating partners have compiled a team of the best minds in the industrial hemp industry to create a totally vertical operation from seed to shelf,” Joshua Weaver, CEO of Sonder Fulfillment, stated in a news release announcing the LOI. “This acquisition by Golden Triangle Ventures will fully capitalize our operations and allow us to further expand our product lines and enter into new markets across the globe.”
- On May 19, 2021, Golden Triangle announced the execution of a formal agreement with Robert “Bo” DuBose to purchase the remaining 49% of HyFrontier Technologies Inc., giving Golden Triangle 100% ownership of the technology company. “This acquisition has been something that Bo and I have been working towards for quite some time and we are both incredibly happy to have this executed,” Dalsgaard stated in a news release announcing the acquisition. “We knew that completing this agreement would show the world that we are both fully committed to our shareholders and the brilliant future of this revolutionary company.”
- On May 12, 2021, Golden Triangle announced its acquisition of a top tier, professional sound system and formed a partnership with SuperKollider Sound LLC to provide a strategic benefit to the company’s entertainment division under Lavish Entertainment Inc. “We are very excited to acquire this unbelievable sound system,” Dalsgaard stated in a news release announcing the acquisition. “Hennessey Sound Design has always been one of my favorite systems on the market, and the team at SuperKollider Sound are true professionals in this space.”
Management Team
Steffan Dalsgaard is the Founder & Executive Chairman of Golden Triangle. He has a background in business development, with over a decade of experience representing and consulting with dozens of private and public companies. Mr. Dalsgaard consults with companies on all of their corporate objectives while providing a professional and corporate face to their organizations. He has built a strong reputation in the public relations industry and has a mission to work with emerging growth companies that are positioned to become significant businesses in their respective fields.
Robert DuBose is the company’s Chief Innovations Officer & Director and the CEO of HyFrontier Technologies Inc. Mr. DuBose is responsible for the success of the HyGrO product in the agricultural market. His experience in the design and production of hydrogen equipment goes back more than a decade, including PEMFC technologies since 2009 with his company, Aquafuel Inc. Mr. DuBose was raised in the farming and machine shop business, where he learned firsthand how much work and love goes into a successful crop, as well as how elements, which are out of the farmers control, can have adverse effects on finances. His belief that being able to deliver a solution to increase growth, yield, health, stamina of crops and profitability for farmers would be a win-win for all led him to create the HyGrO product.
Stuart Seim is the Chief Development Officer & Director of Golden Triangle. He began his career as an associate professor at the University of Manitoba in the field of outdoor and environmental education after receiving his master’s degree and completing advanced educational studies. Coming from a family with an extensive financial background, Mr. Seim became a stockbroker for major regional financial firm Robert W. Baird. In a short time, he became the Branch Manager for Baird in Minneapolis, Minnesota, while also serving as a Managing Director for Baird. During this time, Mr. Seim also served on the board of an industrial hearing company, which he helped to launch as a new company (The TK Group). Mr. Seim currently resides in Colorado, where he is an advisor to several organizations.
Genprex Inc. (NASDAQ: GNPX)
Genprex Inc. (NASDAQ: GNPX) is a clinical-stage gene therapy company developing potentially life-changing technologies for cancer patients based upon a unique proprietary technology platform, including Genprex’s initial product candidate, Oncoprex™ immunogene therapy for non-small cell lung cancer (NSCLC). Genprex’s platform technologies are designed to administer cancer-fighting genes by encapsulating them into nanoscale hollow spheres called nanovesicles, which are then administered intravenously and taken up by tumor cells where they express proteins that are missing or found in low quantities.
Research and Development
Genprex holds a portfolio of 30 issued and two pending patents covering its technologies and targeted molecular therapies. The company’s research and development program is focused on identifying and developing leading-edge gene therapies that can be used alone or in combination with other therapies for treatment of cancer.
Genprex’s initial product candidate is Oncoprex™, an immunogene therapy for the treatment of non-small cell lung cancer (NSCLC). Oncoprex works by interrupting cell signaling pathways that cause replication and proliferation of cancer cells, re-establishes pathways for apoptosis (or programmed cell death) in cancer cells, and modulates the immune response against cancer cells. Oncoprex has also been shown to block mechanisms that create drug resistance.
Preclinical research is being conducted with the goal of developing Oncoprex to be administered with targeted therapies in other solid tumors, and with immunotherapies in NSCLC and other solid tumors. In addition, Genprex has conducted and plans to continue research into other tumor suppressor genes associated with chromosome 3p21.3, as well as other potential applications of the company’s immunogene therapy platform.
Clinical Trials
Genprex is currently conducting the second phase of a phase I/II clinical trial at the University of Texas MD Anderson Cancer Center in Houston. The company plans to expand its clinical program by adding a new clinical study evaluating Oncoprex™ in combination with a checkpoint inhibitor for treatment of Stage IV or recurrent NSCLC. In research presented at the 2017 Annual Meeting of the American Association of Cancer Research in Washington, D.C., Genprex’s collaborators showed that TUSC2 in combination with PD-1 checkpoint inhibition has a significantly greater anti-tumor effect in lung cancer than either agent alone. The research also shows that TUSC2 in combination with PD-1 blockade has synergistic activity in upregulating natural killer (NK) cells, correlating with prolonged survival in mice.
TUSC2 (Tumor Suppressor Candidate 2) is a tumor suppressor gene that is absent or deficient in cancer cells of many different cancer types.
The Market
Genprex technologies seek to bridge a critical gap by combining with targeted therapies and immunotherapies to provide treatments to large patient populations who would otherwise not be candidates for those therapies or who have become resistant to them. Genprex technologies are being developed to overcome genomic limitations which are inherent in targeted therapies and immunotherapies in order to provide new treatment solutions to large cancer populations, such as those with lung cancer.
Each year, more people die of lung cancer than of colon, breast and prostate cancers combined. NSCLC is the most common type of lung cancer, accounting for about 85 percent of all lung cancers, according to the American Cancer Society (“ACS”). Despite radical advances in drug development and novel therapeutic standards, survival for late stage lung cancer has not improved significantly in the past 25 years.
Senior Management
Chairman and Chief Executive Officer J. Rodney Varner, JD, is a co-founder of Genprex and has served in these roles since August 2012. He has more than 35 years of legal experience with large and small law firms and as outside general counsel of a Nasdaq-listed company. Varner has served as counsel in company formation, mergers and acquisitions, capital raising, other business transactions, protection of trade secrets and other intellectual property, real estate, and business litigation. He is a member of the State Bar of Texas and has been admitted to practice before the U.S. Court of Appeals for the Fifth Court and the U.S. Tax Court.
Julien L. Pham, M.D., MPH, is president and chief operating officer of Genprex. In March 2013, Dr. Pham co-founded RubiconMD, a healthcare IT company that connects primary care providers to specialists for additional guidance and opinions on medical cases and served as its chief medical officer. He has served on the faculty at Harvard Medical School’s Brigham and Women’s Hospital and is a board-certified internal medicine doctor and nephrologist.
Ryan M. Confer, MS, has served as Genprex chief financial officer since September 2016. Confer has more than 10 years of executive experience in planning, launching, developing, and growing emerging technology companies and has served in the chief operating and chief financial roles for non-profit and for-profit entities since 2008. Confer has also served as an international business development consultant for the University of Texas at Austin’s IC2 Institute, where he focused on evaluating the commercialization potential of nascent technologies in domestic and international markets applicable to technology incubator programs associated with the University. Confer holds a BS in finance and legal studies from Bloomsburg University of Pennsylvania and an MS in technology commercialization from the McCombs School of Business at the University of Texas at Austin.
Jan Stevens, RN, is vice president of Clinical Operations. Stevens has nearly 20 years of comprehensive clinical operations experience in the biopharma industry and a specialization in early-to-late stage oncology companies. Stevens joined the company to help support the various clinical development programs for Oncoprex™.
Green Hygienics Holdings Inc. (OTCQB: GRYN)
Green Hygienics Holdings Inc. (OTCQB: GRYN) is a California-based innovative technology-driven enterprise focused on the high standard cultivation and processing of industrial hemp and manufacturing of pharmaceutical-grade bioactive cannabinoids.
The company aims to be a leader in compliance and capabilities in the hemp and cannabinoid supply marketplace. By leveraging state of the art technologies, the company intends to open up a whole new world of novel cannabinoids and targeted bio-delivery technologies never before explored, solving the issues of stability, pharmacokinetics, biological tissue penetration and bioavailability.
Dedicated to creating the hemp industry’s safest and finest quality products, the company will be uniquely positioned to deliver product efficacy and supply chain solutions to consumers, as well as to leverage these within its own products and brand portfolio.
USDA Organic Certification and FDA Registration
On August 26, 2020, Green Hygienics registered with the U.S. Food and Drug Administration pursuant to the Federal Food Drug and Cosmetic Act, as amended by the Bioterrorism Act of 2002. This registration strengthens the company’s core mission to provide product efficacy to the pharmaceutical industry and consumers alike.
On September 30, 2020, Green Hygienics was granted USDA Organic Certification (7 CFR Part 205) for the cultivation and post-harvest processing of industrial hemp by the California Certified Organic Farmers for its Sol Valley Ranch property. This certification further enables the company to supply certified organic hemp products to national and international markets.
Market Opportunity
Green Hygienics is focused on finding, acquiring and developing strategically positioned businesses, as well as the best innovations within the hemp industry – a fast-progressing market with remarkable opportunities for growth. The industrial hemp market is expected to reach $5.33 billion in 2020 and is projected to rise to $15.26 billion by 2027, achieving a CAGR of 15.8%, per Grand View Research.
Capital Structure
GRYN has less than 42 million shares outstanding, fully diluted. The company has just 7.2 million common shares in float and boasts a balance sheet with no toxic debt or overhang.
Key Management
Dr. Levan Darjania serves as the company’s Chief Science Officer. Darjania has over 26 years of experience in biotechnology and pharmaceutical drug development. His research and development experience has led him to develop many in-house and collaborative R&D programs over the course of his career.
Kyle MacKinnon serves as GRYN’s Chief Operating Officer. He has extensive knowledge in cannabis processing and was previously the Business Development Manager of Advanced Extraction Systems Inc., a leader in CO2 Supercritical Fluid Extraction. MacKinnon brings over 20 years of sales and management experience to the company.
Ronald Loudoun is the President, CEO, Secretary and Director of Green Hygienics. He received an undergraduate business degree from the British Columbia Institute of Technology. Before joining Green Hygienics, he was the founder and a director of renewable energy firm Archer CleanTech Inc.
Jerry Halamuda is the Senior Vice President of Business Development of the company’s Agriculture Division. He has an extensive career working in the agriculture and horticulture industry. Halamuda has founded, managed and operated multiple successful companies, including Color Spot Nurseries.
John Gildea is GRYN’s Senior Vice President of Corporate Development. He has over 20 years of experience working within the private and public markets. His expertise includes negotiating and structuring private and public financing and mergers. During the course of his work, Gildea has established trusted relationships with a network of equity and capital partners.
GreenBox POS (NASDAQ: GBOX)
GreenBox POS (NASDAQ: GBOX) is an emerging financial technology company leveraging proprietary security and token technology to build customized payment solutions for business. The company’s mission is to build compliant, cutting-edge blockchain ledger tokenized solutions for the diverse, evolving and dynamic global market.
GreenBox applications enable an end-to-end suite of turnkey financial products which offer improved fraud detection and better handling efficiency of large-scale commercial payment processing volumes for its merchant clients globally. The company’s proprietary blockchain and smart contract token technologies create seamless payment processing using digital encryption keys.
GreenBox is a unified platform providing scalability for businesses to accept payments, transact, send, settle and convert in a single versatile ecosystem. GreenBox operates a private and proprietary blockchain-based payment platform that offers distinct advantages when compared to traditional payment technologies, including greater security and data privacy, as well as enhanced identity theft protection and quick settlement.
As the settlement engine for financial transactions, GreenBox’s blockchain technology is a distributed ledger that uses digitally encrypted keys to verify, secure and record details of each transaction conducted within GreenBox’s private ecosystem. The speed and security of the platform allows GreenBox to log immense volumes of immutable transactional records in real time for Tier-1 partners around the world.
In November 2021, GreenBox announced the closing of a previously announced $100 million convertible note financing. The company plans to use proceeds for acquisitions, a planned stablecoin spin-off, and additional working capital toward the company’s future growth. The initial conversion price equals a more than 80 percent premium to the market price of the company’s common stock on October 29, 2021, and values the enterprise at more than $700 million upon conversion.
Brands & Solutions
The company offers multiple solutions and brands under the GreenBox label. The other brands that are nested under the GreenBox POS label include coyni, ChargeSavvy, QuickCard, Transact Europe [didn’t yet close] and Northeast Merchant Systems. Each of these brands play a large role in allowing GreenBox to accel in customizing payment solutions across different verticals and industries.
Payment Solutions
The GreenBox platform offers blockchain secure, robust payment processing solutions for both individual consumers and businesses. The company combines the power and security of blockchain with bank-level tools necessary to both settle transactions and monitor cash flows. Customers can transfer cryptocurrencies like USDC, Ethereum or Bitcoin from external decentralized crypto wallets to their GreenBox wallets. They can also exchange those tokens from their GreenBox wallets to any supported coin. Customers can easily offload in USDC to a debit card or a multitude of gift cards.
White Label Solutions
The company’s white label platform allows it to partner with firms seeking blockchain-based tools to manage merchant relationships. White label partners can monitor cash flows, as well as run reports on merchant transactions, chargebacks, agent and affiliate commissions and more. Partners can access the platform through their partner portal to manage business relationships with full visibility. The platform’s cutting-edge technology saves partners time and simplifies their payment processing. It ensures compliance with automated Know Your Customer and Know Your Bank services and allows customers to set up automated payouts.
coyni Stablecoin
The company is planning soon to launch its own stablecoin, coyni (CYN). coyni is equivalent to the value of the U.S. dollar on a one-to-one ratio. Stablecoin allows for instantaneous transactions with blockchain security just like other cryptocurrency tokens, but without the price volatility of traditional cryptocurrencies. The CYN token is expected to make possible features like digital dollar accounts, cross border payments, international payment processing and other payment solutions. As a smart contract technology, coyni will offer instant settlement using the GreenBox blockchain ledger in any location and currency – crypto or fiat – all at lower fees and in a tokenized secure ecosystem.
Market Overview
A Mordor Intelligence report put the transaction value of the global digital payments market at $5.44 trillion in 2020 and projects the market to be worth $11.29 trillion by 2026. That represents a CAGR of 11.21 percent during the period of 2021-2026.
The report notes that the global COVID-19 pandemic and its impact on e-commerce is likely to encourage strengthened international cooperation and further development of policies for online purchasing and supply. The report states, “The pandemic has made it clear that e-commerce can be an important tool/solution, especially considering the fact that e-commerce sales can support small and medium businesses that form the backbone for certain economies. This is expected to substantially spur the growth of digital payment methods across various economies.”
According to Mordor, other drivers of the growth trend in digital payments include:
- Greater convenience, favorable government policies and evolving consumer behavior worldwide
- Rapid rise in smartphone penetration throughout emerging economies
- Introduction of mobile wallets across the world
- Widespread adoption of retail digital payment services across the vast population of China, serving as a kind of test case for other countries
Management Team
Ben Errez, Chairman of the Board of Directors
Ben Errez’s past positions have included positions at large companies like Microsoft and Intel. He has brought this expertise to lead GreenBox into the forefront of the blockchain-based financial software, services, and hardware market.
Mr. Errez was one of the early managers of Microsoft in 1991. From 1991 to 2004, he served as Software Development Lead for the Microsoft International Office Group. He led the International Microsoft Office Components team (Word, Excel, PowerPoint) in design, engineering, development, and successful deployment. He also served as Executive Representative of Microsoft Office and was a founding member of the Microsoft Trustworthy Computing Team both within the company and internationally. Mr. Errez co-authored the first Microsoft Trustworthy Computing Paper on Reliability. At Microsoft, he was responsible for the development of the first Microsoft software translation Software Development Kit (“SDK”) in Hebrew, Arabic, Thai, and Simplified Chinese, as well as the development of the first bidirectional extensions to Rich Text Format (“RTF”) file format and all bidirectional extensions in text converters for Microsoft Office. He also contributed to the development of the international extensions to the Unicode standard to include bidirectional requirements under the World Wide Web Consortium (“W3C”).
In 2004, Mr. Errez transitioned into the world of consulting, where he held the position of Principal Consultant from founding to the present date, through which he advises clients in the South Pacific region with market capitalizations ranging from $50 million to $150 million on commerce, security, reliability, and privacy.
In 2017, immediately before partnering with Fredi Nisan to launch GreenBox, Mr. Errez was asked to take over the Microsoft Alumni Network for the Southern California region as a regional director. Mr. Errez has been a principal of GreenBox since its inception in 2017.
Fredi Nisan, Chief Executive Officer
Fredi Nisan’s career in technology began during his years of service in the Israeli Defense Forces, where he served as IT Manager for all of Israel’s Northern Bases. After serving in the military, Mr. Nisan opened and operated a computer hardware store before becoming the Inventory Operations Manager for Zicon Israel in 2005, a hardware and software producer. At Zicon, he supervised inventory operations, worked on quality controls for motherboards and chips, and educated customers on software and hardware product functionality. Subsequently, Mr. Nisan moved to the United States, where he worked for One Coach in San Diego, California, as a business coach. One Coach specializes in customized growth solutions for small business owners, including the latest strategies for sales, internet marketing, branding, and ROI. Mr. Nisan was consistently ranked as the top salesperson for small business coaching while working with One Coach.
In 2010, Mr. Nisan launched Brava POS, where he served as President until 2015. Brava POS provided point of sale (“POS”) systems for specialty retail companies. Mr. Nisan developed software to provide clients with solutions for issues ranging from inventory management to payroll to processing high volume transactions in the form of a cloud-based POS system. This system had the capability to manage multiple stores with centralized inventory and process sales without an internet connection, and offered a secure login for each employee, as well as including advanced inventory management and reporting, plus powerful functionality for its end users.
In 2016, Mr. Nisan founded Firmness, LLC. Through Firmness, he created “QuickCitizen,” a software program that simplifies the onboarding process for new clients of law firms specializing in immigration issues. The QuickCitizen software significantly reduced law firms onboarding processing time from more than three hours to approximately 15 minutes. Mr. Nisan has been a principal of GreenBox since its August 2017 inception. In January 2018, Firmness sold QuickCitizen to GreenBox.
Jacquline B. Reynolds, Chief Marketing Officer
Jacqueline B. Reynolds is the company’s Chief Marketing Officer. She served most recently as vice president of marketing for Sprouts Farmers Market. She has built her reputation as a world-class global marketer, working with Coca-Cola, McDonald’s, Verizon, Walmart, L’Oréal, Xbox, 7-Eleven and many other Fortune 500 brands. She has managed award-winning marketing programs with partners such as the NFL, Super Bowl LIV, the Olympics, the FIFA World Cup, Sony Pictures, Universal Music and others.
Cepton Inc. (NASDAQ: CPTN)
Cepton Inc. (NASDAQ: CPTN) is a provider of state-of-the-art, intelligent, lidar-based solutions serving a range of markets, including automotive (ADAS/AV), smart cities, smart spaces and smart industrial applications. General Motors (NYSE:GM) has granted a series production award for Cepton’s lidar, the biggest such award to date in the automotive space. Cepton’s is the lidar component of GM’s Ultra Cruise autonomous driving platform. By leveraging its patented Micro Motion Technology (MMT®) lidar platform, the company develops reliable, scalable and cost-effective solutions that deliver long-range, high-resolution 3D perception for smart applications.
Cepton was established in 2016 by co-founders Dr. Jun Pei and Dr. Mark McCord. The company is headquartered in San Jose, California, and serves a fast-growing customer base through an international presence spanning North America, Germany, Japan, India and China.
Micro Motion Technology (MMT®)
Cepton was built from the ground up to meet key lidar industry challenges for mass market adoption. This company’s portfolio of proprietary technology is uniquely aimed at facilitating this industry growth through a combination of performance, reliability, affordability and design integration.
Key among its innovations is MMT®, a mirrorless, frictionless, rotation-free 3D imaging platform designed specifically for lidars. Its benefits for OEMs and system integrators include:
- Reliability – The durable design uses common, easily attainable materials.
- Versatility – The platform is capable of achieving near- to ultra-long range with a wide field of view.
- Efficiency – MMT® features a compact form factor, low power usage and inexpensive components.
- Scalability – Its simple design means that scale-up to high manufacturing volumes is easily attainable.
Because of their compact form factor, Cepton lidars are embeddable and ideally suited for advanced driver-assistance system (ADAS) integration, whether behind windshield, in headlamp or in fascia.
Agreement with KOITO
KOITO Manufacturing Co. Ltd., the world’s premier Tier 1 auto lighting supplier, originally started an evaluation of Cepton’s MMT® based lidars in 2018. In 2020, KOITO made an investment in Cepton aimed at accelerating the company’s development and enabling KOITO’s industrialization of high-performance and high reliability lidar sensors for ADAS and autonomous vehicle (AV) applications.
Through this collaboration, Cepton was able to secure the largest ADAS lidar series production award[1] with General Motors as a sole source in the automotive space. The award covers GM vehicles for the initial period of 2023-2027.
On August 5, 2021, the two companies deepened their relationship when KOITO committed to invest a further $50 million in Cepton’s business through its participation in a Private Investment in Public Equity (PIPE) offering of shares of common stock of Growth Capital Acquisition Corp. in connection with Cepton’s recent merger.
Collaboration with GM
On July 13, 2021, Cepton announced that it had secured an ADAS lidar series production award from a leading, Detroit-based global automotive OEM – the biggest lidar production award by any OEM to any lidar company. It was later clarified that the OEM was General Motors, and Cepton’s lidar is part of GM’s ADAS Ultra Cruise system.
GM is “expected to deploy Cepton lidars in its next generation of advanced driver assistance systems (ADAS) across multiple vehicle classes and models – not just luxury cars.” As such, the agreement marks the potential for “an industry-first, mass-market adoption of lidar technology for automotive ADAS, with an anticipated deployment in consumer vehicles starting in 2023.”
On July 28, 2021, Ford Motor Company (NYSE: F) distributed an article on Medium noting, “Ford has been engaged with Cepton almost since their inception in 2016, both for R&D collaboration and small-scale deployments. Cepton LiDAR are deployed in some of [Ford’s] smart city projects. Based on Ford’s guidance, Cepton delivered a custom version of their LiDAR to enable R&D on advanced ADAS features.”
Market Outlook
Driven by increasing development and adoption in automobile safety applications, environmental mapping and 3D-modeling, the global lidar market is forecast to experience considerable growth over the coming years. A research report published by MarketsAndMarkets suggests that the sector will grow to an estimated $3.4 billion by 2026, achieving a CAGR of 21.6% over the next five years.
The report further highlights increasing investments in lidar startups by automotive giants as a driver of growth opportunities in the sector, particularly in North America.
In 2020, ground-based lidar accounted for the lion’s share of the overall lidar market, and this trend is expected to continue as the automotive sector continues to rapidly advance adoption across the full spectrum of vehicle classes. One factor not to be underestimated is the high barrier of entry and the exceptionally long time required for automotive OEMs to vet and award a production win to a lidar company. It is a commonly held view that the over 50 lidar companies will inevitably coalesce into a handful serving all OEMs.
Cepton, having a head start through its established partnership with leading global OEM GM, is uniquely positioned to capitalize on this market growth in the years to come.
Management Team
Cepton’s founder-led team is made up of lidar industry pioneers with decades of collective experience across advanced lidar and imaging technologies.
Jun Pei, Ph.D., is the company’s CEO and Co-Founder. He is a technology specialist with a focus in optics and electronics. Prior to founding Cepton, Dr. Pei founded AEP Technology, a firm focused on developing advanced 3D optical instruments. He received his Ph.D. in electrical engineering from Stanford University.
Mark McCord, Ph.D., is Cepton’s CTO and Co-Founder. Prior to founding Cepton, he led advanced development at KLA-Tencor. Dr. McCord also formerly served as an associate professor at Stanford University, where he earned his Ph.D. in electrical engineering.
Winston Fu, Ph.D., is the company’s CFO. Dr. Fu is the founder of Silicon Valley venture capital firm LDV Partners. Prior to joining Cepton, he served as CFO and Chairman of Active-Semi before its acquisition. Dr. Fu has also helped to build many technology companies as an entrepreneur and/or board member. He received his Ph.D. in applied physics from Stanford University, as well as an MBA from the Kellogg School of Management at Northwestern University.
[1] Largest known ADAS lidar series production award based on number of vehicle models awarded
Hemptown USA

Hemptown USA, headquartered in Central Point, Oregon, is a proven grower of full-spectrum hemp biomass grown using premium seed genetics that contain less than 0.3% THC and exceptionally high cannabinoid (CBD) content of up to 20%. The company's "soil to oil" methodology combines seasoned professionals working in hand-picked agricultural microclimates located in Oregon's famed Emerald Triangle, Kentucky and Colorado.
Hemptown has exclusive rights to 1 million rare CBG (cannabigerol) seeds genetically programmed to yield from 15% to 20% full-spectrum non-intoxicating cannabinoids. As a result of a long-standing relationship with the one of the world's most respected cannabis breeding companies – Oregon CBD Seeds – Hemptown is positioned to be a leading CBG producer in the U.S. in 2019 and beyond.
In 2018 Hemptown's harvest from its Oregon hemp farm was 150,000 pounds of full-spectrum biomass with CBD content hovering around 17%. 2018 harvest revenue expected to range from $8.1 million to $12.6 million. The company is scaling up operations in 2019 to meet market demands and projects it will reap over 1,000,000 pounds. By 2020, Hemptown projects potential revenues in the $100 million to $200 million range are possible once additional farming operations are at full strength.
Growth Strategy
By 2020, Hemptown anticipates it will have more than 3,000 acres in several states dedicated to hemp farming. Expansion plans include increasing in-house extraction capabilities to boost profit margins by providing additional CBD and CBG isolates and distillation services. Development of business-to-business channels as well as new products and formulations for the direct-to-consumer market, along with several strategic acquisitions, are also key to Hemptown's growth strategy.
Hemptown plans to expand distribution and growing operations globally through strategic partnerships and development of contracts with leading Fortune 500 brands in European markets. The company intends to grow its IP portfolio by developing a proprietary water-soluble cannabinoid delivery system. Not to be confused with water-compatibility, water-soluble cannabinoids combine seamlessly with other liquids, have a superior shelf life, and deliver dramatically increased efficacy to the consumer.
Branded Products
Hemptown's first in-house branded product line combines the inspiring strength found in the unbridled nature that surrounds the company's original hemp farm in the Siskiyou Klamath region of Oregon. Siskū is set to redefine the cannabinoid packaged goods space with an elegant look, clean feel and potent, reliable efficacy.
Custom product lines can also be created for any product manufacturer as Hemptown brings GMP and ISO accredited processing facilities online in 2019. Together with Oregon CBD Seeds and Hemptown's product sciences team, Hemptown will be able to create custom, proprietary full-spectrum CBD and CBG oils and pure isolates.
Management Team
Company Chairman Rod Wolterman founded Hemptown's Oregon operations in 2016. He has extensive experience in the cannabis sector having been active within the space since 1998. Wolterman has also acted as a private equity investor in numerous medical marijuana dispensaries and cultivation operations in southern California.
CEO John Cummings has over 20 years of experience in finance, marketing, sales and project management. He led the compliance and special projects efforts for Kings Garden, one of the largest vertically integrated operators in California. Cummings also spent a year in Europe launching the continent's first GMP and ISO-accredited cultivation and manufacturing facility.
Dr. Gordon Chiu is chief science officer for Hemptown USA. He has more than 15 years of combined domestic and international experience in biomedical, chemical, cosmetic, medical and technology industries. A graduate of Rensselaer Polytechnic Institute with a master's degree from Seton Hall University, Chiu is leading Hemptown's cannabinoid research team and is responsible for filing IP patents, specifically in the areas of water-solubility, bioavailability and peptide sequencing.
Hero Technologies Inc. (OTC: HENC)
Hero Technologies Inc. (OTC: HENC) is a cannabis company with a vertically integrated business model and plan that includes cannabis genetic engineering, farmland for medical and recreational cannabis cultivation, production licenses, distribution licenses, consumer packaging, retail operations and dispensaries that make the organization a multi-state operator (MSO).
The company was founded in 2004 and is headquartered in Dover, Delaware.
Portfolio
The company holds the majority stake in BlackBox Systems and Technologies LLC, an aeroponic cannabis cultivation firm focused on providing optimal conditions to enhance photosynthesis and cultivation. Hero Technologies is planning expansion in cultivation and dispensary operations in Colorado through wholly owned subsidiary Mile High Green LLC, while expansion in Massachusetts is planned through another wholly owned subsidiary, MassCannabis LLC.
Hero Technologies also owns and operates HighlyRelaxing.com under Highly Relaxing LLC and recently acquired the assets of V Brokers LLC, now operating as Veteran Hemp Co. at VeteranHempCo.com.
BlackBox Systems and Technologies LLC
BlackBox Systems and Technologies LLC markets a proprietary cannabis aeroponic cultivation system designed for the large-scale production of top-shelf cannabis products. BlackBox offers the optimal conditions to enhance photosynthesis and promote the cultivation of large flowering plants. The system’s dry room, process room and secure storage were designed for precise control through each phase of the cannabis lifecycle. Weekly harvests are achieved using 13 separate BlackBox systems in independent modules.
The system provides a series of key benefits, including:
- High-pressure nutrient delivery, with no nutrient or PH deficiencies
- Sterile, 100% nutrient solution
- Drain to Waste (no reuse of wastewater)
- Low water usage (1 gallon per plant per day)
- Constant PH and EC in reservoirs
- Modular design (1 to 100 pods in any configuration)
- Innovative proprietary engineering
- Minimal cleanup
- Media-less growing, suspended in the air, with no media waste
- No pesticides
Highly Relaxing LLC
Highly Relaxing LLC is an emerging Henderson, Nevada-based operation dedicated to providing customers with honestly labeled, high-quality hemp-derived CBD products. Its current offerings include a topical CBD cream that provides localized relief from potential discomfort.
Veteran Hemp Co.
Veteran Hemp Co.’s mission is to provide a quality, consistent and delicious product for Americans looking to enjoy the hemp smoking experience. Its product is brought in by only the finest farming operations delivering the best genetics. Veteran Hemp Co. has its own custom harvest plans, drying facilities and all of the logistics that fall between. Veteran Hemp Co. prides itself on being a veteran-approved company.
Market Outlook
The global legal cannabis market is anticipated to reach $84 billion by 2028, expanding at a CAGR of 14.3% from 2021 to 2028. The driving factor for this forecast expansion is the increasingly widespread legalization of cannabis for medical and recreational use. Recreational use accounted for 60.3% of industry revenue in 2020.
North America provided the largest revenue share in the cannabis market, accounting for 91.1% of the global market in 2020. Due to the early legalization of medical and recreational cannabis in the region, the customer pool has increased exponentially (https://nnw.fm/snpHh).
The global CBD market was valued at $2.8 billion in 2020 and is expected to grow at a CAGR of 21.2% and reach $13.4 billion by 2028. North America is considered the most progressive region for cannabis and its derived products, with the highest number of CBD companies being based on the continent. The B2B (business to business) segment dominates the CBD industry, accounting for the largest revenue share at 59.6% in 2020 (https://nnw.fm/cGxXQ).
With its vertically integrated business model and development into a multi-state operator across multiple sectors of the cannabis industry, Hero Technologies is uniquely positioned to capitalize on the fast-growing market and the growing number of opportunities emerging as a result of legalization and increased popularity among consumers.
Management Team
Gina Serkasevich, CPA, CMA, is the Chief Executive Officer, Treasurer and Secretary of the Hero Technologies. She previously worked for Holloman Corporation as its Director of Finance beginning in June 2012 and was appointed Chief Financial Officer of Holloman Energy Corporation in August 2014. She has more than 30 years of domestic and international corporate accounting and finance experience. She served as U.S. Controller for EFLO Energy Inc., a company focused on the acquisition, exploration and development of oil and gas assets in North America. Prior to 2012, Ms. Serkasevich worked in the oil and gas tanker transportation industry as a Regional Financial Manager for AET Inc. Limited (2011-2012), as a Financial Consultant for OSG Ship Management Inc. (2009-2011) and as a Financial Controller/CFO for Stena Bulk LLC (1998-2008). During her 11-year tenure at Stena Bulk LLC, she established the financial, accounting and reporting requirements for its new joint ventures and tanker pools with Sonanagol USA and held the Company Secretary position on both of those companies’ boards of directors.
Dan McCarthy is the company’s Corporate Development Manager. He has spent more than 12 years in the institutional investment community, holding various investment banking and private equity executive roles. Thus far, he has been a part of over $1 billion in transactional value ranging from debt and equity to acquisitions and diversities throughout his career. Mr. McCarthy’s most recent role was Managing Director at Petro Capital, a Dallas-based private equity and investment bank. He began his career working for a private international consulting firm based in Washington, D.C., helping corporations and funds expand into non-G7 countries utilizing World Bank financing. He is also a graduate of the University of Kansas School of Business and completed the Mergers and Acquisitions program at the New York Institute of Finance.
James Rowland is Hero Technologies’ Marketing Advisor and an expert in marketing and e-commerce. He has held many high-level marketing and business-related roles. He is the Founder and current CEO of PerfectCheckout.com and the current Business Development Specialist at Fulfillment.com. Mr. Rowland has held multiple high-level positions throughout his career, which have provided him with the experience needed to bring success-backed marketing leadership skills to his current role with the company.
Hillcrest Energy Technologies Ltd. (CSE: HEAT) (OTCQB: HLRTF)
Hillcrest Energy Technologies Ltd. (CSE: HEAT) (OTCQB: HLRTF) (FRA: 7HIA.F) is a clean technology company based in Vancouver, British Columbia, engaged in developing high-value, high-performance power conversion technologies and digital control systems for next-generation powertrains and grid-connected renewable energy systems.
From concept to commercialization, Hillcrest invests in the development of energy solutions that power a more sustainable and electrified future. Hillcrest power inverter technology helps produce efficiencies in electrification and maximize the performance of electric systems, including electric vehicles (EV), motors and generators.
The company offers a flexible, single-inverter architecture that can be applied at nearly every stage of the electrification ecosystem, from renewable energy generation through the charging and operation of an EV, to provide full-cycle efficiency and performance improvements.
As momentum to electrify and decarbonize energy systems accelerates, Hillcrest believes the power inverter is increasingly emerging as a key component. While system cohorts such as battery packs, PV panels and electric motors are often in the spotlight, the inverter holds the key to unlocking efficiency and performance improvements.
Hillcrest power inverter technology is:
- REVOLUTIONARY: high-efficiency inverter technology has the potential to revolutionize how motors respond and how efficiency is gained.
- AGILE: able to deliver and deploy high-efficiency inverter solutions purpose-designed to meet specific customer needs.
- INNOVATIVE: technology-forward, clean-energy experts who are focused on advancing and optimizing efficient alternative energy use across all electric vehicle and charging platforms.
- A MARKET LEADER: a next-generation technology provider to the automotive industry’s top suppliers and manufacturers.
Technology & Applications
Hillcrest’s first application for its inverter technology – a 250 kW|800V Hillcrest SiC high efficiency traction inverter – is focused on the growing EV market. Hillcrest technology eliminates traditional design trade-offs faced across the power industry – deploying higher switching frequencies has historically meant a greater increase in losses, lower system efficiency and higher heat. Through a combination of hardware and software expertise, Hillcrest enables power applications to leverage higher switching frequencies AND
- Realize improved power system performance and reliability
- Operate at higher power levels without compromising efficiency
The expected benefits of Hillcrest’s traction inverter have been confirmed via testing and shared in a technical white paper, published in April 2022, that confirmed the following results:
- Significant efficiency gains – 99%-plus inverter efficiency
- Increased power density targeting 50kW/L+
- Significantly increased motor efficiency
- Lower stress on mechanical and electrical parts, enhancing reliability
- Improved thermal management
Hillcrest has also filed a patent for an enhanced powertrain solution that offers the potential to simplify EV charging and redefine how the industry envisions charging infrastructure. The company believes the most exciting benefit of the enhanced powertrain solution is the ability to eliminate the onboard charger and booster from an EV, as well as faster, anywhere charging including direct DC, wireless, and bidirectional charging across current and future power levels. Hillcrest sees this as a true EV charging game changer.
The company’s technology applies to nearly every clean energy industry segment:
- Wind power – an inverter is deployed at a wind turbine generator to convert the AC output, with at least one additional inverter used to deliver the power to the grid/battery.
- Solar power – an inverter is used to convert the DC output from the photovoltaic panels into the AC power that flows to the grid/battery/home.
- Energy storage – an inverter is deployed to convert the DC output from the storage system or batteries to the AC power that flows to the grid/home/EV.
- EV fast chargers – an inverter converts the AC input from the grid/storage system to the DC output needed to charge an EV’s battery.
Market Outlook
According to an April 2022 market analysis by Vantage Market Research (VMR), the global power inverter market is expected to reach a value of $95 billion by 2028, driven by increasing demand for EVs, energy generating wind turbines and solar-powered photovoltaic systems. That jump is forecast from an estimated $70.5 billion market value in 2021 and represents a compound annual growth rate of more than 5%.
According to the VMR report, many governments in countries around the world are supporting alternative options for efficient and nonpolluting energy generation. This has boosted demand for wind energy and solar energy systems. Hillcrest is aiming to capture a share of this future market growth across nearly every segment of the clean energy industry.
Management Team
Don Currie is the founding CEO of Hillcrest Energy Technologies. He has led the company’s successful transition from fossil fuels into clean energy technologies. Earlier in his career, he held various senior level positions, including director, officer and vice president of corporate communications with Enhanced Oil Resources Inc., an oil and gas exploration and production company based in Houston. Prior to that, he worked in other private and public ventures spanning the mining, gaming and technology sectors.
Jamie L. Hogue is the COO of Hillcrest. She brings more than two decades of progressive policy leadership, economic analysis and organizational development experience to Hillcrest. She builds collaborative processes and solutions that drive growing organizations toward a more resilient future. She previously served as the director of operations for Arizona State University’s Ten Across initiative – a compelling observatory positioned on the front lines of economic, social and climate change. She earned a master’s degree in public administration and a bachelor’s degree in economics from Arizona State University.
Ari Berger is Chief Technology Officer at Hillcrest. He brings over a decade of commercial experience with a track record of deploying new electrification technologies and go-to-market strategies. In 2015, he founded NIG Systems Ltd. in Israel, which specializes in custom high performance control systems design. Prior to this, he previously worked for Bental Industries, a leading motor manufacturer. He holds a master’s degree in system control engineering from the Technion – Israel Institute of Technology.
Raj Clair is CFO at Hillcrest. She is a CPA who began her career at Deloitte and has served in advanced finance positions in the energy and resources sector. She has been responsible for reporting, audits and internal controls, as well as working on budgeting and forecasting. She has worked with various publicly listed companies, including SEC registrants, and has both Canadian and U.S. experience. She holds a bachelor’s degree in accounting from Simon Fraser University.
Home Bistro Inc. (OTC: HBIS)
Home Bistro Inc. (OTC: HBIS) is a Miami-based company engaged in the business of providing prepackaged and prepared meals to consumers. The company has created the next generation of prepared meal delivery – Ready-Made Gourmet Meal Delivery 3.0.
Home Bistro addresses the three major problems facing the prepared food delivery market: poor food quality; customers tired of eating the same meals; and, eating at home is still eating at home, with the accompanying food preparation and clean up chores. The company addresses these problems by delivering high quality food fresh and fast, providing customers a variety of meal choices from a diverse lineup of celebrity chefs, and requiring simple prep and easy clean up without sacrificing the fine dining experience.
Home Bistro offers a family of high quality, direct-to-consumer, ready-made, gourmet meals. Using the latest fresh food “skin-packing” technology, Home Bistro offers a virtual “Bistro Emporium” where consumers can cross select from a wide variety of siloed “bistros,” each with a dedicated section and unique visitor experience created by a renowned celebrity/executive chef. Meals delivered fresh can be eaten within 10 to 14 days or frozen for up to six months.
The company’s mission is to lead the next generation of heat-to-eat food delivery with unique and delicious cuisine and an experience that excites the market. Home Bistro’s advantage in the highly competitive meal delivery space is meal diversity – with the best celebrity chefs from around the world, offering a home-based fine dining experience through a selection of over 50 unique gourmet meals, as well as offering a developing selection of desserts and single-serving wine to perfectly complement the meal experience. In addition, the company uses only the highest quality ingredients in its meals and preserves their freshness by employing state-of-the-art vacuum skin packing.
In mid-2021, Home Bistro acquired southern-California based Model Meals, a lifestyle ready-to-eat meal prep service, which is Whole30 and Paleo approved, while then only serving three states. In September 2021, Home Bistro commenced shipping Model Meals to all 50 states and recently announced that it will launch a subscription-based service for Model Meals consisting of three meals per day (breakfast, lunch and dinner) for up to five days per week. The subscription service, expected to launch by May 2022, will initially target the Southern California market, where Model Meals maintains a food production and fulfillment facility and enjoys a strong customer base.
Brands and Products
Home Bistro’s leading online platform (www.homebistro.com) provides direct-to-consumer, heat-to-eat, celebrity chef-inspired gourmet meals. Offerings currently include inspirations developed by “Iron Chef” Cat Cora, two-time New York Times best-selling cookbook author and TV host Ayesha Curry, sports-tailgating focused creator of “Hungry Fan” Chef Diana Falk, “Master Chef” Claudia Sandoval, and “Top-Chef All-Star” Richard Blais. Soon-to-launch celebrity chefs on the Home Bistro platform include “Caterer to the Stars” Roblé Ali, “zero-waste cooking” celebrity chef Priyanka Naik, and CHOPPED champion Melanie Moss.
Home Bistro’s Model Meals lifestyle brand (www.modelmeals.com) is a Whole30 and Paleo approved, ready-to-eat meal prep service, offering a weekly rotating menu that is prepared by professional chefs, using only the highest quality ingredients available, sourced responsibly and locally, and delivered in sustainable, eco-friendly packaging.
Home Bistro has partnered with celebrity chef Melanie Moss to expand its dessert menu options. In keeping with its mission to deliver a complete gourmet culinary experience to discerning customers, Home Bistro beta-tested its first dessert – a delicious, sweet and salty caramel brownie. Based on the encouraging results, the company is moving forward to create a much more robust dessert menu.
Home Bistro has formally launched its wine offering initiative with In Good Taste Wines, a unique direct-to-consumer wine platform that empowers wine lovers to “discover the world, by the glass.” The company has worked diligently with the In Good Taste Wines team to develop a unique selection of elegant single-serving wines to pair with Home Bistro’s celebrity chef-inspired meals. The partnership with In Good Taste Wines provides Home Bistro with a low-cost, incremental source of revenue, which will assist the company in expanding its gross profit margin and lead it to faster profitability.
Market Outlook
Global revenue in the online food delivery sector was $136 billion in 2020 and forecast to grow steadily at a 7.5% CAGR through 2024 to a projected value of $182 billion.
In the U.S., the food delivery sector, which comprises both the restaurant-to-consumer segment and the platform-to-consumer segment where Home Bistro operates, is expected to surpass $32.3 billion in 2024. The company’s addressable market, the platform-to-consumer segment, is approximately 30% of the U.S. market and is projected to reach a value of $9.7 billion by 2024. This segment is expected to grow even faster than the sector as a whole as providers refine their focus on healthier meals, more convenient delivery and subscription options and more advanced meal processing technology.
Management Team
Zalmi Duchman is Chairman and CEO at Home Bistro. He was CEO and founder of The Fresh Diet online meal delivery service, which grew from a startup to over $30 million in annual revenue. He is a thought leader, investor and publisher of numerous articles in the food tech sector. He was named one of Forbes “America’s Most Promising CEOs Under 35,” and was named a Miami Herald “20 Under 40” entrepreneur in 2014.
Carlo Ricci is Director of Operations at Home Bistro. He was VP Operations for The Fresh Diet online meal delivery service, where he developed the culinary and R&D departments and established distribution centers in five states. He was also Operations Manager at Homemade Meals, where he developed and implemented inventory systems, established production facilities on both coasts and trained and managed personnel. He has a bachelor’s degree in data analytics from Miami Dade College.
Camille May is CFO at Home Bistro. She is a co-founder of Model Meals meal delivery service, where she has served as CFO since the company’s inception in 2015. She helped build the company from the ground up to more than $2 million in annual revenue. Prior to Model Meals, she worked as a financial analyst and broker in commercial real estate. She has a BBA in finance from the Leeds School of Business at the University of Colorado.
Danika Brysha is Chief Marketing Officer at Home Bistro. She co-founded Model Meals and was also a co-founder of the Self-Care Society. She is a former fashion model and founder of Danika Brysha Inc., a service specializing in modeling, coaching, speaking, events, media and influence. She is creator of the Brunch Series and a Whole30 certified coach. She is also host of the top-rated podcast “Light + Life Live” and is a lifestyle design expert. She earned a bachelor’s degree from the University of Colorado.
iClick Interactive Asia Group Ltd. (NASDAQ: ICLK)
iClick Interactive Asia Group Ltd. (NASDAQ: ICLK) is an independent online marketing and enterprise data solutions provider connecting worldwide marketers w/ith audiences in China. Built on cutting-edge technologies, iClick’s proprietary platform possesses omni-channel marketing capabilities and fulfills various marketing objectives in a data-driven and automated manner, helping international and domestic marketers reach their target audiences. Headquartered in Hong Kong, iClick operates in 10 locations worldwide, including Asia and Europe.
iClick aims to become a fully integrated Enterprise and Marketing Cloud Platform in China, providing clients a full consumer-cycle solution. This is facilitated by two pillars’ growth strategy through two business segments: Marketing Solutions and Enterprise Solutions.
Marketing Solutions
Using data and AI-driven technology to help brands efficiently identify, target and acquire the right customers
As the leading programmatic marketing platform in China, iClick’s proprietary platform collects a wealth of data from multiple sources to precisely reach the right audience at the right moment, on the right channel and right device. Cross-screen search solutions capture critical micro-moments when users proactively search for what they need. This multi-dimensional approach to marketing allows iClick to effectively understand internet users and exponentially widen target audiences for its brand clients. Multiple monetization models available in the Marketing Solutions segment allow iClick to serve its clients in several ways, such as audience targeting.
Data-driven marketing is indispensable to marketers targeting specific audiences in China. More than 825 million internet users in China are anonymously profiled on iClick’s platform, which boasts cross-channel and cross-screen capabilities.
Enterprise Solutions
Enabling brands to efficiently manage their consumers through online and offline data integration and analysis, increase the repurchase rate, and enhance consumers’ loyalty
iClick’s Enterprise Solutions segment addresses enterprise needs in China, particularly focusing on “smart retail,” an expanding and innovating market involving the combination of online and offline consumers’ behavioral information. Enterprise Solutions support detailed profiling of customers, which facilitates data-driven business strategies, enhances business processes at various levels, and increases operational and marketing efficiency.
Enterprise Solutions leverages iClick’s proprietary platform that incorporates Artificial Intelligence (AI) to learn, build and store knowledge, enabling accurate predictions about consumer behavior that ultimately provide marketing solutions derived from the large amount of available data.
Through a strategic partnership with Tencent, iClick’s Enterprise Solutions presents strong recurring revenue streams with tremendous opportunities to upsell multi-national corporations (MNCs). Tencent’s proprietary API connection enables brands to build 360-degree consumer profiles based on the collection and integration of purchased behavioral information from online and offline touchpoints, including WeChat Mini Programs, WeChat Payment, WeChat Work and more.
As iClick continues to provide integrated marketing and smart retail solutions targeting Chinese consumers, the company believes Enterprise Solutions has strong long-term growth potential and will become a major gross margin contributor in the future.
Partnerships
In 2019, iClick established various agreements and partnerships with a number of leading southeast and northeast Asian companies for regional diversification and in 2020 is focused on continuing to develop additional partnerships and new business models globally. Many of the world’s top companies are leveraging iClick’s proprietary data platform to precisely identify and reach out to core target audience groups in China.
The company’s partnerships include:
- A tri-partnership with BTG WELINK, an online retail services arm of Beijing Tourism Group (“BTG”), and Tencent Holdings Ltd., China’s leading provider of internet value added services. As part of this partnership, iClick applies its upgraded solutions to build a private DSP (Demand Side Platform) system for BTG. Using Tencent’s big data advertising platform, iClick can assist BTG to develop precision marketing campaigns.
- An Advertising Agency Authorization Certificate from Baidu Inc. (NASDAQ: BIDU), under which iClick is designated the authorized agency for native advertising of Baidu’s news feed ads. Native advertising is a consumer-friendly, non-disruptive advertising format that has gained rapid popularity among advertisers in recent years. Native advertising and creative marketing content have become a more effective marketing method among the Chinese young consumers. In 2019, the native advertising sector was estimated to have an around 53.5% share of the online advertising revenue, according to Statista.
- A joint-venture partnership with VGI Global Media Plc (VGI.BKK), Thailand’s No. 1 online to offline (O2O) solutions provider across advertising, payment and logistics platforms, which enables brands in Southeast Asia to capture the multi-billion-dollar Chinese consumer market through a range of technology-driven marketing solutions.
Case Study: Armani Hotel Dubai
Dubai has been gearing up to welcome the growing wave of Chinese visitors. Chinese nationals are eligible for a 30-day visa-on-arrival into the UAE, which gives Chinese travelers tremendous convenience. In light of this, Armani Hotel Dubai set the objective to increase its sales in this market.
The challenge: What Aarmani Hotel Dubai lacked in executing this goal was insightful understanding of Chinese travelers in particular the demographics that were likely to be attracted to the hotel. Challenged by the huge differences in the business practice, unique culture and language barrier in running digital campaigns in China, Armani Hotel Dubai turned to iClick’s know-how and expertise to guide its campaign to success and meet its sales goal.
The solution: iClick tailored an optimal solution for the hotel to increase brand awareness and booking rate from China – which is the key market for the hotel – and successfully assisted Armani Hotel Dubai in reaching its target Chinese audiences by using China’s most popular mobile and internet sites, including WeChat and Weibo, to improve reach and booking potential.
The results: Due to iClick’s unrivaled technological and execution strengths, Armani Hotel Dubai’s ads were delivered in an omnichannel manner, raising brand awareness and garnering interest between Chinese consumers. Subsequently, Armani Hotel Dubai saw a surge in conversion rate.
During the campaign, the Armani Hotel Dubai brand was connected with 87% of Chinese mobile users.
Award-winning Provider
iClick, a Deloitte Technology Fast50, has received multiple industry awards from the international marketing community. The company is committed to helping clients access digital China with its omni-channel, data-driven marketing solutions that deliver uniquely sharpened marketing capabilities and outstanding advertising results.
Most recently, iClick subsidiary OptAim (Beijing) Information Technology Co., Ltd was recognized by Tencent Ads as a 2019 Gold Service Provider. Tencent Ads also named OptAim the winner of three major annual awards for the second half of 2019: “Outstanding Contribution of the Year,” “Best Technology & Data Application Award,” and “Best Branding Awards.”
In November 2019, company co-founder and CEO Sammy Hsieh was chosen as the winner of the “EY Entrepreneur of The Year China 2019 Award in Technology Category,” an award recognizing his entrepreneurial acumen, innovative spirit and strong leadership. As one of the world’s most prestigious business accolades, the “EY Entrepreneur of The Year” awards program honors those who accomplish success by combining ability with opportunity, and inspire others with great vision, leadership and outstanding achievement.
iClick won the Annual Influential Platform Award and the Innovation Golden Award in Marketing at the Creative Award 2019, as well as the Best Tourism Marketing Agency. The company was also the recipient of the “Best Brand and Performance Marketing Award” at the Performance Marketing Ecosystem Summit 2018 hosted by the Advertising & Marketing Service, a division of Tencent Holdings Limited.
The company in 2018 was also recognized as “Platinum Service Partner of Tencent Social Ads” at the Tencent Key Accounts Mid-Year Summit held in Beijing. The mobile division of iClick, Optaim, received the same award beginning in 2016. Optaim was also the “Best DSP Partner” and “Key Account Data Partner” of Tencent, making it the only player in China with such unique and deep level of cooperation with Tencent Social Ads.
Leadership
Sammy Wing Hong Hsieh, chairman of the board and co-founder, was CEO from 2009 to 2019. Prior to co-founding iClick, Hsieh held senior positions in several prominent technology companies. He was general manager for Asia Pacific at Efficient Frontier (now an Adobe company), a leading digital performance marketing company, and was director of Search Marketing at Yahoo Hong Kong from 2000-2008. Hsieh received a bachelor’s degree in economics from the University of California, Los Angeles.
Jian Tang, director, CEO and co-founder, has 20 years of experience in digital advertising and is well-known in China for his expertise in advertising technologies and big data. In 2012, he founded OptAim, which was acquired by iClick in 2015, and has served key research, engineering and management roles at Yahoo’s global research and development center. Tang received his doctorate in computer engineering from Tsinghua University and was named by Campaign Asia as one of the leaders in its Digital A-List in 2016.
Terence Chi Wai Li, chief financial officer, has 15 years of experience in financial management, investment and business operations. He has served in management roles and advisory capacities at several start-ups, in addition to financial management and fundraising roles. He previously worked at PricewaterhouseCoopers, specializing in M&A due diligence and cross border tax and deal structuring projects. Li received an MBA from Oxford University’s Said Business School. He is a Fellow Member of ACCA, a Member of HKICPA, and a Chartered Financial Analyst.
Ideanomics Inc. (NASDAQ: IDEX)
Ideanomics Inc. (NASDAQ: IDEX) is a global company facilitating the adoption of commercial electric vehicles and supporting next-generation financial services and fintech products. Ideanomics is currently divided into two divisions – mobility and capital. These divisions provide shareholders with access to disruptive and high-growth opportunities.
The company expects 2021 to be another growth year after it raised approximately $400 million over the past six months. This funding has already been put to good use with acquisitions of Wireless Advanced Vehicle Electrification (WAVE) and Timios. With roughly $200 million still on the balance sheet, Ideanomics continues to look for new investments and acquisitions in revenue-based opportunities focused on EV and fintech businesses.
Founded in 2004, Ideanomics is headquartered in New York, New York, with additional offices in Hangzhou, Beijing and Qingdao, China. Its current operations span the United States, China, Ukraine and Malaysia.
Ideanomics Mobility
Ideanomics Mobility is focused on the EV market. The global commercial EV market was valued at $34.7 billion in 2018 and is expected to grow at a CAGR of 39.9% through 2022 to reach a total of $132.73 billion (https://ibn.fm/pPrf4). According to a survey by Grand View Research, the global EV charging infrastructure market is also expected to grow and reach $144.97 billion in 2028, expanding at a CAGR of 33.4% from 2021 to 2028.
This growth is expected to be driven by increased support of electric vehicles from the public, as well as the current U.S. administration, which has a goal of achieving a 100% clean-energy economy.
The Ideanomics Mobility unit consists of five companies:
- Mobile Energy Global (MEG) – Wholly owned China-based service provider of the Sales-to-Finance-to-Charging (S2F2C) business model to assist commercial fleet operators on EV enablement. Recent sales include 2,000 units of D1, BYD’s custom electric ride-hailing vehicle.
- Medici Motor Works – Wholly owned North America division. MMW will develop zero-emissions specialty vehicles, trucks, buses and vans for the North American market.
- Wireless Advanced Vehicle Electrification (WAVE) – Wholly owned Utah-based commercial EV charging technology company with a specialized offering of in-ground wireless charging for commercial vehicles. WAVE’s chargers power the Antelope Valley Transportation Authority, the largest municipal EV bus system in the country. Its revenue for 2020 exceeded $7 million, and it boasts a robust pipeline for 2021 and beyond.
- Treeletrik – Majority investment in Malaysian-based OEM will service a high-demand market – electric delivery mopeds. Treeletrik has obtained certifications in Thailand and Indonesia, with orders secured for 2021. Its North American marketing program is expected to commence in 2021. As a part of the ESG initiative, one tree will be planted for every unit sold.
- Solectrac – Minority investment in California-based electric tractor company. Solectrac manufactures 100% electric tractors to benefit farmers, crops and the planet at a time when the agriculture market remains virtually unaddressed by EV solutions.
- Silk EV – Minority investment in hyper car and performance car design company, which provides access to the high-end battery and charging technology development ecosystem.
Ideanomics is generating EV revenue from its Sales to Financing to Charging (S2F2C) business model, which features three operating areas:
- Vehicle and Battery Sales: Medici, Treeletrik and Solectrac cover three key market segments
- Financing, Leasing and Insurance: Offering financial services to fleet customers, commission delivery and origination fee-based revenue
- Charging and Energy Services: Offering charging as a service, battery swap programs and WAVE wireless charging products
Ideanomics Capital
Ideanomics Capital is focused on providing disruptive fintech solutions across the entire board of financial services, ranging from financial markets to digital securities and assets to mortgages and more. More mainstream institutions and a growing number of companies have increased their digital securities services, along with institutional investments boosting bitcoin and the emergence of favorable regulatory developments, creating ample opportunities for widespread adoption of financial technologies.
Additionally, the U.S. real estate industry is ripe for technologization, as it currently is fragmented, antiquated, opaque and largely untouched by tech innovation. However, the expanding market, with U.S. home sales expected to grow 21.9% in 2021, and the increased digitization of all business spaces are expected to promote a digital-first experience as the new industry standard this year and beyond (https://ibn.fm/DwsUv).
The Ideanomics Capital unit consists of five companies:
- Timios – Wholly owned subsidiary bringing real estate into the 21st century by providing value-add, fee-based services addressing the title and closing process of home buying and mortgage transactions. Timios works to create transparency and efficiency within the market. Timios ended 2020 as a cash flow and EBITDA positive business.
- The Delaware Board of Trade (DBOT) – Wholly owned FINRA-regulated ATS and broker dealer based in Delaware.
- Liquefy – Minority investment bringing innovation to investment in real assets with blockchain technology by increasing efficiency in fractional ownership, lowering entry to investment barriers and unlocking liquidity in assets that were previously illiquid.
- Technology Metals Market (TM2) – Minority investment in UK company delivering a direct investment and trading market for technology metals with a newly accessible technology metals asset class for inventory diversification. The traded metals are 100% backed by physical metals.
- Intelligenta – Investment providing AI and machine learning solutions for financial institutions and regulators.
Management Team
Alf Poor is Ideanomics’ Chief Executive Officer. He is a client-focused and profit-driven executive who has a track record of success in rapidly growing technology companies and large, multi-national organizations. Mr. Poor’s expertise includes business planning, financing and creating and implementing corporate governance policies, as well as handling management across organizations. His specialization is working with cross-border and multi-national startups. Before taking the CEO role at Ideanomics, he was the CEO for Global Data Sentinel.
Conor McCarthy is the company’s Chief Financial Officer. He is a strategic and operationally oriented management-level professional. His extensive international experience is within the fintech, data science and advertising technology sectors. Mr. McCarthy has experience with public companies, PE, and VC-backed firms. His specializations are financial and management reporting, planning and analysis, financial modelling, performance metrics, KPIs, venture borrowing, Series A equity funding, ERP system implementation, international business operations, and acquisition due diligence and integration. Before joining Ideanomics, Mr. McCarthy most recently held a CFO position at OS33. Prior to that, he was CFO for Intent Media Inc.
Kate Lam is the company’s Managing Director of Financial Products. She is highly regarded for her fixed income capital marketing skills across Asia and the United States. Ms. Lam has over 25 years of experience in the financial markets industry, dealing with many asset classes and clients. Having spent a few years in the fintech startup industry, her skills bridge the gap between traditional financial assets and new technological innovations. She has held senior management positions at Bear Sterns, Deutsche Bank and Standard Chartered Bank.
Keith Byers is Ideanomics’ Senior Vice President of Operations. He has extensive experience managing strategic relationships with key clients and deepening the relationships through innovation and successful engagement strategies. Before Ideanomics, Mr. Byers was the Managing Partner and Head of Operations for Gain Theory. He has a Master of Arts – MA, Economics from Heriot-Watt University and a Master of Science – Economics from The University of Edinburgh.
Tony Sklar is the company’s Senior Vice President of Investor Relations. He is a communication strategist and has worked for multi-faceted companies with global operations. Mr. Sklar handles omni-channel distribution using intelligence platforms and data insights for strategic planning, international expansion and marketing channels. His specialties include project management with digital strategy and transformation, ICO, marketing, blockchain and strategic partnerships. In addition to his role with Ideanomics, he is also a board member for the Delaware Board of Trade and the host and senior technology reporter for Far From TV.
Infobird Co., Ltd (NASDAQ: IFBD)
Infobird Co., Ltd (NASDAQ: IFBD) is a software-as-a-service (SaaS) provider of AI-powered customer engagement solutions in China. Infobird leverages a self-developed cloud computing structure, AI and machine learning capabilities, patented Voice over Internet Protocol (VoIP) application technologies, a no-code development platform and in-depth industry expertise to best serve its growing client base.
Founded in October 2001, Infobird empowers clients with value-driven business solutions designed to increase revenue, reduce costs and enhance service quality and customer satisfaction. The company currently specializes in corporate clients in finance and a broad array of ancillary industries.
Infobird is headquartered in Beijing, China, and began trading on the Nasdaq Capital Market on April 20, 2021, following an initial public offering of 6.25 million ordinary shares at a public offering price of $4.00 per share, before underwriting discounts and commissions.
Product Offering
Infobird’s flagship customer engagement software can handle both AI Customer Engagement and AI Salesforce Management.
- AI Customer Engagement
- Intelligent Omni-Channel Customer Service – This offering allows clients to connect with their customers anytime and anywhere through a comprehensive suite of cloud-based tools.
- Cloud Call Center – This service puts Infobird’s years of technical and operational experience to work for clients, with options including intelligent IVR technology, call monitoring, routing strategy and ticketing systems, all supported by multi-dimensional data reports.
- Intelligent Telemarketing – Infobird’s AI bots can help clients navigate “never-ending lists” of potential customers, filter out the most promising leads and increase the working efficiency of agents, keeping agents focused on high-value tasks.
- AI Voice Chatbot and AI Text Chatbot – This technology allows clients to create human-like interactions offering 24/7 availability and multi-round dialogue capabilities, decreasing labor costs by up to 80% while greatly improving efficiency.
- AI Salesforce Management
- Intelligent Quality Inspection – Infobird’s platform aims to improve quality inspection rates and service levels through the use of real-time smart monitoring with comprehensive coverage.
- Intelligent Training – Interactive training programs allow clients to ensure and continuously improve the performance level of their agents, lessening the impact of high turnover rates common throughout the customer service industry.
Infobird’s client base includes roughly 10,000 paid user accounts representing 358 customers in the industries of finance, education, public services, consumer products and health care – as reported on June 30, 2020.
Market Outlook
Cloud infrastructure services spending in China increased by 32% ($39.9 billion) in the fourth quarter of 2020. For all of 2020, total services grew to $142 billion, up from the reported $107 billion in 2019. This growth can be attributed to rising demand for cloud infrastructure over physical software solutions (https://ibn.fm/rHZUh). China is the second-largest market for cloud infrastructure solutions after the U.S., accounting for roughly 14% of the global industry.
Likewise, SaaS has demonstrated considerable growth potential in recent years. In 2020, the SaaS industry in China was valued at $3.3 billion, representing an increase of 43.5% over 2019, as companies continue to leverage artificial intelligence and Big Data technologies to increase efficiencies and promote expansion.
As one of the leading and longest standing providers of domestic SaaS solutions and with a comprehensive portfolio of intelligent, customizable and scalable solutions, Infobird is uniquely positioned to capitalize on the market’s expansion and resulting opportunities for corporate growth.
Management Team
Yimin Wu is the CEO and Founder of Infobird. He has served as the Chairman of the board of directors and Chief Executive Officer of the company since it was founded. From August 1990 to March 1993, Mr. Wu was a software engineer for the Software Center of Tsinghua University and was sent to the U.S. to co-develop the HP_UX operating system at HP Inc. From April 1993 to May 2000, he served as the general manager for Beijing Jing Zhou Computers Co. Ltd., a company responsible for marketing and developing interactive voice response systems. From July 2000 to October 2001, Mr. Wu was the general manager for Beijing Jing Zhou Rong Hua Internet Technology Co. Ltd, a company responsible for developing middleware for call center establishments. He received a bachelor’s degree and a master’s degree in computer sciences from Tsinghua University.
Hsiaochien Tseng is the EVP of Infobird and has held the title since January 2020. From March 2010 to September 2018, he served as a sales director for the Credit Card Center of China Guangfa Bank, where he was responsible for integrating and managing online and offline sales channels, establishing overall and regional sales strategies and creating training systems to increase the client base. From October 2018 to January 2020, Mr. Tseng served as SVP of Hua Tuo Digital Technology Group Co. Ltd., a financial information technology company. He received a bachelor’s degree in information management from Fu Jen Catholic University and a master’s degree in business administration from San Diego State University.
Chunhsiang Chen is the VP of Infobird, a position he has held since April 2012. From June 1990 to February 1993, he served as an advisory programmer of International Business Machine Corp. (IBM). During that time, he participated in the design and development of the Multiple Protocol Transport Network. From February 1993 to September 1996, Mr. Chen served as an associate professor in the Information Education Department of National Taiwan Normal University. He founded GenNet Technology Co. Ltd., an information technology company, in 1993 and served as the president until joining Infobird in 2012. Mr. Chen has a bachelor’s degree in computer sciences from the National Chiao Tung University and a master’s degree and doctoral degree in computer sciences from Northwestern University.
Lianfang Zhou is the CFO of Infobird and has been with the company for over 10 years. From September 2004 to July 2008, she served as the head of accounting at Beijing Saishuo Technology Co. Ltd., a software development company specializing in port services. From August 2008 to December 2009, Mrs. Zhou served as the head of accounting for Beijing Lianhe Lida Investment Co. Ltd., a property management services company. She holds an intermediate accounting qualification certificate issued by the Ministry of Finance of the PRC. Mrs. Zhou also has a bachelor’s degree in accounting from the Renmin University of China.
InMed Pharmaceuticals Inc. (NASDAQ: INM)
InMed Pharmaceuticals Inc. (NASDAQ: INM) is a global leader in the manufacturing and clinical development of rare cannabinoids. InMed is a clinical stage company developing cannabinoid-based pharmaceutical drug candidates, as well as manufacturing technologies for pharmaceutical-grade rare cannabinoids.
The company is dedicated to delivering new therapeutic alternatives to treat conditions with high unmet medical needs. The company is also developing a proprietary manufacturing technology to produce pharmaceutical-grade rare cannabinoids in the lab and has recently announced an LOI to acquire a leading rare cannabinoid manufacturer.
Research and Technology
There are more than 100 rare cannabinoids found in only trace amounts in the cannabis plant, together making up less than 1% of the plant’s biomass. InMed is initially focused on the therapeutic benefits of cannabinol (CBN) in diseases with high unmet medical need. Preclinical studies of CBN demonstrated an excellent safety profile and showed CBN has potential for therapeutic benefit over other cannabinoids such as tetrahydrocannabinol (THC) and cannabidiol (CBD).
Evidence suggests there may be great therapeutic potential in rare cannabinoids. Each has a specific chemical structure, and different cannabinoids have been observed to have distinct physiological properties in humans, including therapeutic potential for specific diseases as well as unique safety profiles. CBN is the active pharmaceutical ingredient (API) in InMed’s two lead programs for dermatological and ocular diseases.
InMed’s most advanced compound, INM-755, is a CBN topical cream under clinical development for the treatment of epidermolysis bullosa, a severe genetic skin disorder. To date, INM-755 has been evaluated in two Phase 1 clinical trials in healthy volunteers. InMed has filed Clinical Trial Applications in several countries as part of a global Phase 2 clinical trial of INM-755 (cannabinol) cream in epidermolysis bullosa. Responses from the National Competent Authorities and Ethics Committees are expected throughout the summer of 2021.
InMed is also involved in developing INM-088, an ocular CBN formulation being researched for the treatment of glaucoma, the second leading cause of blindness in the developed world. InMed is currently evaluating several formulations to deliver CBN into the eye to address issues of dosing frequency, side effects and treatment penetration. INM-088 is being designed for topical delivery to the eye. This localized delivery results in very little drug being absorbed or migrating into the bloodstream, thus minimizing potential adverse side effects. INM-088 shows promise to reduce intraocular pressure and provide neuroprotection of the eye.
Manufacturing
The limited availability of rare cannabinoids like CBN makes them economically impractical to extract directly from the plant for pharmaceutical use. InMed is developing IntegraSyn, a cannabinoid synthesis manufacturing system to create rare cannabinoids in the lab that are bioidentical to the compounds derived from the cannabis plant. IntegraSyn uses multiple standard pharmaceutical processes and has achieved a cannabinoid yield of 5 grams per liter, surpassing commercial viability and significantly exceeding currently reported industry yields. InMed is now focusing on manufacturing scale-up to larger batch sizes while continuing process optimization, targeting increased cannabinoid yield and further reducing overall cost of goods.
BayMedica Inc. Acquisition
On June 29, 2021, InMed announced it had entered into a non-binding letter of intent to acquire BayMedica Inc., a private company based in Nevada and California that specializes in the manufacture and commercialization of rare cannabinoids.
As noted in the news release, BayMedica is a revenue-stage biotechnology company leveraging its significant expertise in synthetic biology and pharmaceutical chemistry to develop efficient, scalable and proprietary manufacturing approaches to produce high quality, regulatory-compliant rare cannabinoids for consumer applications. BayMedica is currently commercializing the rare cannabinoid CBC (cannabichromene) as a B2B supplier to distributors and manufacturers marketing products in the health and wellness sector. BayMedica is planning additional rare cannabinoid launches for the coming year.
Pursuant to the indicative terms of the LOI, InMed and BayMedica intend to negotiate and enter into a definitive agreement under which InMed would acquire 100% of BayMedica in exchange for 1.6 million InMed common shares to be issued to BayMedica’s equity and convertible debt holders, with any such issued InMed common shares being subject to a six-month contractual hold period.
Market Outlook
There is a rapidly growing demand for rare cannabinoids. However, their low natural concentration makes traditional harvesting of these compounds cost prohibitive. Biosynthesis allows production of rare cannabinoids in the lab that are bioidentical to compounds found in nature, with significantly higher yields which reduce costs. Biosynthesis can produce pharmaceutical-grade, bioidentical, THC-free compounds at a cost that’s 70 to 90 percent less than wholesale prices of naturally harvested rare cannabinoids.
Cannabinoid-based pharmaceuticals are expected to overtake the market as rare cannabinoids become less expensive and more available. According to Statista, the value of the consumer market for cannabinoid-based pharmaceuticals in the United States is forecast to grow to $25 billion by 2025 and to $50 billion by 2029, with cannabinoid-based pharmaceuticals used to treat health conditions including pain, respiratory conditions, autoimmune conditions and more.
Management Team
Eric A. Adams has been CEO and president of InMed since June 2016. He has more than 25 years of experience in establishing corporate entities, capital formation, global market development, mergers and acquisitions, licensing and corporate governance. He previously served as CEO at enGene Inc. Prior to enGene, he held senior positions in global market development with QLT Inc. (Vancouver), Advanced Tissue Sciences Inc. (La Jolla, CA), Abbott Laboratories (Chicago, IL) and Fresenius AG (Germany).
Bruce S. Colwill is InMed’s CFO. He has more than 25 years of financial leadership experience in public and private companies. Prior to InMed, he served as CFO of General Fusion Inc., a private clean energy company. He was also CFO at Entrée Resources Inc., a mineral exploration company, from 2011 to 2016. He has held CFO roles at Neuromed Pharmaceuticals Ltd., Response Biomedical Corp, Forbes Medi-Tech Inc. and Euronet Worldwide Inc.
Alexandra D.J. Mancini is Senior Vice President, Clinical and Regulatory Affairs at InMed. She has more than 30 years of global biopharmaceutical research and development experience. She has been an executive with numerous biotech companies, including senior vice president of Clinical and Regulatory Affairs at Sirius Genomics; senior vice president of Clinical and Regulatory Affairs at INEX Pharmaceuticals; and vice president of Regulatory Affairs at QLT Inc.
Eric C. Hsu is Senior Vice President, Pre-Clinical Research and Development at InMed. He joined InMed with more than 18 years of scientific leadership experience in the field of gene therapy. He has held various positions within enGene Inc., including vice president of Research and vice president of Scientific Affairs and Operations. He received his Doctorate from the Department of Medical Biophysics at the University of Toronto.
Michael Woudenberg is Vice President, Chemistry, Manufacturing and Controls at InMed. He has more than 20 years of successful drug development, process engineering, GMP manufacturing and leadership experience. He has held positions with 3M, Cardiome Pharma, Arbutus Biopharma and, most recently, was Managing Director of Phyton Biotech LLC.
InnerScope Hearing Technologies Inc. (OTC: INND)
InnerScope Hearing Technologies Inc. (OTC: INND) is a Nevada corporation incorporated on June 15, 2012, with its principal place of business in Roseville, California. The company was initially started in 2006 – operating as InnerScope Advertising Agency Inc. – to provide advertising and marketing services to retail establishments in the hearing device industry. On August 25, 2017, the company changed its name to InnerScope Hearing Technologies Inc. to better reflect its current direction as a hearing health technology company that manufactures, develops, distributes and sells numerous innovative hearing health-related products, hearing treatments and hearing solutions, direct-to-consumer (DTC) through a scalable business model.
The company is a manufacturer and a distributor/retailer of DTC, FDA (U.S. Food and Drug Administration) registered, Bluetooth app-controlled hearing aids and personal sound amplifier products (PSAPs), hearing-related treatment therapies, doctor-formulated dietary hearing supplements, proprietary CDB oil for treating tinnitus and assorted hearing and health-related products targeting approximately 70 million Americans suffering from hearing-related problems. The company’s mission is to improve the quality of life of the 70 million people in North America and the 1.5 billion people worldwide who suffer from hearing impairment and/or hearing-related issues.
The management team of InnerScope is applying decades of industry experience and believes it is well-positioned, with its innovative in-store point-of-sale Free Self-Check Hearing Screening Kiosks (“Hearing Kiosks”), to directly benefit when the Over the Counter (OTC) Hearing Aid Act (the “OTC Hearing Aid Law”) is enacted (expected in late 2021 based on the President’s Executive Order issued on July 9, 2021) The OTC Hearing Aid Law allows OTC hearing aids for perceived mild-to-moderate hearing losses to be sold in retail stores without having to see a professional. InnerScope’s Hearing Kiosk is designed to help the tens of millions of Americans with undetected/untreated mild-to-moderate hearing loss treat themselves with the company’s easy, convenient and affordable OTC hearing aids, in-store and/or online.
Industry Game-Changer – New Emerging Market with 48 Million Potential Customers
The following is sourced from The White House Fact Sheet detailing an Executive Order from President Biden aimed at saving Americans with hearing loss thousands of dollars by allowing hearing aids to be sold over the counter at drug stores:
“Hearing Aids: Hearing aids are so expensive that only 14% of the approximately 48 million Americans with hearing loss use them. On average, they cost more than $5,000 per pair, and those costs are often not covered by health insurance. A major driver of the expense is that consumers must get them from a doctor or a specialist, even though experts agree that medical evaluation is not necessary. Rather, this requirement serves only as red tape and a barrier to more companies selling hearing aids. The four largest hearing aid manufacturers now control 84% of the market.”
On July 9, 2021, President Biden noted the following in reference to his Executive Order relating to hearing aids:
“Right now, if you need a hearing aid, you can’t just walk into a pharmacy and pick one up over the counter. You have to get it from a doctor or a specialist. Not only does that make getting hearing aids inconvenient, it makes them considerably more expensive, and it makes it harder for new companies to compete, innovate and sell hearing aids at lower prices.”
“As a result, a pair of hearing aids can cost thousands of dollars. That’s a big reason why just one in seven Americans with hearing loss actually use a hearing aid.”
InnerScope Game-Changers
For InnerScope, this Executive Order could present a significant opportunity. The company is uniquely positioned with a number of strategic advantages and offerings in the space, including:
- First to Market: Free self-check hearing screening kiosks deployed in national pharmacy chains, big-box retailers & national and local groceries chains
- Online Hearing Screening Tests: For national retailers to use their websites to attract more customers in conjunction with the company’s in-store hearing kiosks
- The HearIQ App for iOS and Android users: Offers a free self-check hearing test and provides a user control function for InnerScope’s Bluetooth app-controlled self-adjusting rechargeable hearing devices
- Customer Monthly Subscription Model: Offering the lowest, most affordable monthly payment options (as low as $42 per month for pair of rechargeable, app-controlled hearing aids) for consumers to purchase hearing aids and receive free upgrades every two years.
The In-Store Hearing Screening Kiosks and Online Free Hearing Screening Tests
Innerscope’s hearing screening kiosk and online hearing screening tests offer free self-check hearing evaluation using the world’s first “Hearing Triage” artificial intelligent pattern recognition software, which has a unique ability to classify both level (degree of loss) and pattern (type of loss). In addition, the software can detect the probable location of the hearing problem and its degree of severity.
The tests are developed as a hearing wellness tool to help track hearing ability and (if tests results indicate a hearing loss) make recommendations for in-store point of sale or online purchase of one of InnerScope’s hearing devices, as well as providing recommendations to see one of the professionals in InnerScope’s local contracted network of hearing health care experts for further follow-up testing if necessary. The software also generates an audiometric report which is instantly emailed to the customer.
The HearIQ App
InnerScope is the creator of the HearIQ App, which offers free self-check hearing tests and provides a user control function for InnerScope’s line of Bluetooth app-controlled self-adjusting rechargeable hearing devices. InnerScope developed the free hearing test part of the HearIQ App to help with the early detection of hearing loss for the 1.5 billion people worldwide who have untreated hearing loss or some form of hearing issues that may be undetected and do not have access to a computer for InnerScope’s online hearing screening test.
Hearing Aid Products
Through its dedicated online store, MyHearIQ.com, InnerScope offers affordable, direct-to-consumer, Bluetooth app-controlled, self-adjusting hearing technology to empower consumers to take control of their hearing care. InnerScope’s hearing technology allows the customer in less than 10 minutes using any smartphone to personalize each hearing device to their hearing needs using an onboard in-ear custom-fit self-testing feature through the HearIQ App.
InnerScope is shifting hearing health care from traditional brick-and-mortar hearing care clinics to customers’ homes by providing a unique solution to give customers top quality, affordable access to hearing aids without the need to see a hearing professional or go to a hearing care clinic. As a result, InnerScope can deliver the same level and quality of hearing technology and expert support for the customer from their homes at a fraction of the cost of traditional channels. All InnerScope hearing aid devices are medical-grade and available with professional remote programming and support services from one of the company’s licensed hearing professionals through the HearIQ App.
Hearing & Tinnitus Dietary Supplements
InnerScope has developed a proprietary line of doctor-designed hearing & tinnitus dietary supplements to help people with hearing problems protect themselves from future hearing issues. There are currently three types of formulas to choose from, including Ear-Ring Relief for the 60 million Americans who suffer from tinnitus, HearingVite + Memory Boost for people with hearing loss and cognitive issues, and HearingVite + Multivitamin for maintaining proper hearing health and levels of nutrients.
Complete Line of Hearing Health Care Products
InnerScope offers a brand label of assorted ear care and hearing aid maintenance products. In support of overall ear health and ensuring maximum performance from its hearing aids and comfort for its customers, InnerScope provides a whole line of care items, including cleaning kits, wipes, spray and drying tablets, ear cleaner for wax removal, a natural lubricant agent for new hearing aids and hydrating lubricating ear gel.
Verified Wholesale and Direct-to-Consumer Sales
InnerScope is a verified wholesaler with Walmart for premium affordable direct-to-consumer hearing aids, personal sound amplification and hearing health accessories. InnerScope also created an easy shopping experience for its hearing and tinnitus vitamins through Walmart and Amazon Prime. With new partnerships in the works, the company aims to add other online and brick-and-mortar establishments to its vitamin distribution network in the future.
Hearing Aid Market Outlook
The global hearing aid market is expected to reach $11.02 billion by 2028, growing at a CAGR of 7.4% during the forecast period. This marks a significant increase from the $6.47 billion value reported in 2020, an increase largely driven by innovations being made in hearing aid technology (https://ibn.fm/bRWUb).
As a leading wholesale provider and direct-to-consumer business, InnerScope is positioned to disrupt the global hearing aid market. Its partnerships with some of the United States’ largest retail distributors and wholesalers are only strengthening the company’s position within the industry.
Management Team
Matthew Moore is the President and CEO of InnerScope Hearing Technologies Inc. He grew up in the hearing health industry, working alongside his grandfather through internships and mentorships. At the age of 10 years old, he became Chief Marketing Officer and Chief Operating Officer of his parent’s private hearing aid practice, the largest in Northern California and the second largest in the state. Matthew has shown his leadership ability by creating distribution partnerships with big industry names and independent retailers/pharmacies.
Kim Moore is the Chief Financial Officer of InnerScope Hearing Technologies Inc. She has worked in the hearing aid industry for over 45 years, helping her father maintain his hearing aid practice in Central Valley, California. She began working on marketing with her father at the age of eight, learning that no customer walks through the door without proper advertising and marketing. As a licensed hearing instrument specialist, Kim has given hearing tests to more than 30,000 people.
Mark Moore is the Chairman and Co-Founder of InnerScope Hearing Technologies Inc. He has over 35 years of experience in hearing aid dispensing, practice management, private label brand management and hearing aid marketing. He has personally fit hearing aids to over 10,000 hearing-impaired people. In addition, he has been responsible for developing and testing proven new industry marketing and advertising methods and best practice strategies, which has made him one of the most sought-after experts in the hearing aid industry. Mark was previously a columnist for Advanced for Audiologists, a global industry publication, and served on the American Academy of Audiology (AAA) advisory board for AudiologyNow conventions. He has also developed patented and patent-pending nutritional supplements for hearing-related issues, aural rehabilitation programs and low-level laser therapy for tinnitus and sensorineural hearing loss.
Knightscope, Inc. (NASDAQ: KSCP)
Knightscope, Inc. (NASDAQ: KSCP), founded in 2013 and based in Mountain View, California, is a leader in the development of autonomous security capabilities targeting to disrupt the $500 billion security industry. Knightscope’s technology uniquely combines self-driving technology, robotics, artificial intelligence and electric vehicles.
Knightscope designs and builds Autonomous Security Robots (ASRs) that provide 24/7/365 security to the places you live, work, visit and study. The company’s client list covers public institutions and commercial business operations, including multiple Fortune 1000 companies to date. These ASRs have been proven to enhance safety at hospitals, logistics facilities, manufacturing plants, schools and corporations. ASRs act as highly cost-effective complementary systems to traditional security and law enforcement officials, providing an additional advantage by continuing to offer uninterrupted patrolling capabilities across the country.
The company’s ASRs have assisted in the arrest of suspects involved in crimes ranging from armed robbery to hit-and-runs. Their machine-embedded thermal scanning capability even aided in preventing the breakout of a major fire. You can learn more about the crime fighting wins at www.knightscope.com/crime
The company has achieved several milestones since its creation in 2013, including:
- Establishing itself in a 15,000-square-foot facility located in Mountain View, California, in the heart of Silicon Valley, where Knightscope designs, engineers and builds its technology (Made in the USA)
- Operating for more than 1 million hours in the field and securing contracts across five time zones, from Hawaii to Rhode Island
- Raising over $100 million since inception to build its technology from scratch and generating over $13 million in lifetime revenue, validating both the market opportunity and the technology
Growth Capital & Proposed Nasdaq Listing
With backing from more than 28,000 investors and four major corporations and over $100 million raised since inception, Knightscope is poised to be an industry leader in the future of public safety and security.
On December 1, 2021, Knightscope announced the commencement of an offering of up to $40 million of its Class A common stock, with shares to be listed immediately following closing on the Nasdaq Global Market under the ticker symbol ‘KSCP’. The offering is for up to 4 million shares priced at $10 per share. Learn more at www.knightscope.com/investors
Company Mission – Reimagining Public Safety
Knightscope’s long-term vision has an eye on the greater good. The company’s mission is to make the United States of America the safest nation in the world while supporting the 2+ million law enforcement and security professionals across the country.
Crime has an estimated negative economic impact in excess of $2 trillion annually. As crime is reduced, positive impacts will likely be realized across several aspects of society, including housing, financial markets, insurance, municipal budgets, local business and safety in general.
Knightscope CEO William Santana Li was interviewed by Kevin O’Leary, more commonly known as Shark Tank’s Mr. Wonderful. When asked to explain how the benefits provided by the ASRs outrank a human doing the same job, Li said, “First, just the simple presence of a physical deterrent causes criminal behavior to change. Second, the machines are self-driving cars that patrol all around and recharge themselves. They also generate 90 terabytes of data per year. No human would ever be able to process that. The robots are intended to be eyes and ears for the humans, not a one-to-one replacement.”
The Knightscope solution to reduce crime combines the physical presence of ASRs, sometimes referred to as proprietary Autonomous Data Machines, with real-time onsite data collection and analysis. The ASRs are fitted with eye-level 360° cameras, thermal scanning, public address announcements and various other features that work in tandem with humans to provide law enforcement officers and security guards unprecedented situational awareness.

Those 90 terabytes of data are then formatted in a useable way, so law enforcement can leverage that information and execute their responsibilities more effectively.
Public Safety Innovation
The company’s recurring revenue business model is set up to mimic the recurring societal problem of crime, and it takes into consideration the fact that innovation in the security and public safety industry has been stagnant for decades. Because the traditional practices of the sector have remained unchanged for years, automation has potential to drive substantial cost savings – and significant improvement in capabilities.
Human security guards are one of both the largest expenses and the largest liabilities for companies. Knightscope’s robots are offered at an effective price of $3 to $9 per hour, compared with approximately $85 for an armed off-duty law enforcement officer and $15 to $35 for an unarmed security guard.
This innovation has the potential to drive considerable cost savings. Based on these estimates, manufacturing costs can be recovered as soon as the first year of operation.
Product Offerings
The company has nine patents and a framework of unique intellectual property. Knightscope currently offers a K1 stationary machine, a K3 indoor machine and a K5 outdoor machine. A K7 multi-terrain four-wheel version is in development.

The ASRs autonomously patrol client sites without the need for remote control, providing a visible, force multiplying, physical security presence to help protect assets, monitor changes in the area and deter crime. The data is accessible through the Knightscope Security Operations Center (KSOC), an intuitive, browser-based interface that enables security professionals to review events generated by the ASRs providing effectively ‘mobile smart eyes and ears’. Learn more at www.knightscope.com/ksoc
The ASRs and the related technologies were developed ground up by the company and are Made in the USA.
The Robot Roadshow

Knightscope has created the ultimate hybrid physical and virtual event, bringing its Autonomous Security Robot technologies to cities across the country for interactive and in-person demonstrations.
Each roadshow landing is hosted virtually by a Knightscope expert, and visitors can interact directly with each of the company’s ASRs and see the Knightscope Security Operations Center (KSOC) user interface in action. Learn more at www.knightscope.com/roadshow
Management Team
Chief Executive Officer William Santana Li is a veteran entrepreneur, a former executive at Ford Motor Company and the founder of GreenLeaf, a company that grew to be the world’s second-largest automotive recycler and is now part of LKQ Corporation (NASDAQ: LKQ).
Chief Client Officer Stacy Dean Stephens brings his experience as a former Dallas law enforcement officer, as well as his skills as a seasoned entrepreneur, to assist on the client acquisition side.
Chief Intelligence Officer Mercedes Soria is an award-winning technologist and former Deloitte software engineer.
Chief Design Officer Aaron Lehnhardt brings over two decades of two- and three-dimensional product and industrial design in modeling and VR to the table, on top of his experience as a senior designer at Ford Motor Company.
Chief Financial Officer Mallorie Burke is a seasoned financial executive and strategic advisor for both private and publicly traded technology companies with a successful track record of mergers & acquisitions, corporate growth and exit strategies, including public listings.
General Counsel Peter Weinberg leverages 30 years of diverse corporate counsel experience, spanning from startups to well-established companies, private and public. He has significant experience training personnel at all levels in critical areas to improve corporate compliance and productivity.
Kronos Advanced Technologies Inc. (OTC: KNOS)
Kronos Advanced Technologies Inc. (OTC: KNOS) develops and sells a variety of disruptive, advanced, state-of-the-art air filtration and purification systems that fully remove harmful allergens, bacteria, viruses (including the flu), and even gasses from indoor breathing spaces, including healthcare and other settings.
Kronos’ own patented medical-grade technology is tested as the most effective clean air solution on the market. Kronos filters particles down to .0146 micron (.0146μm) – far beyond the 3 microns (0.3μm) of a traditional HEPA filter. Kronos® not only collects but destroys air pollutants. Kronos® AIR 5G® Air Purifiers use about 30,000 volts inside to actively destroy 99.99% of all airborne bacteria, mold, and virus particles.
Kronos® devices operate silently using nanotechnology to remove 100% of pollutants in a 400ft2 room (up to the whole house) and replenishes the room with pure, clean air every 15 minutes. Indoor household air is often four times more polluted than outdoor air, and Kronos air purifiers act like bionic lungs for the home and protect the people in it.
Unlike traditional HEPA systems that collect pollutants on filters which can, over time, grow mold and bacteria, Kronos’ patented technology destroys and eliminates all manner of harmful particles and deposits them on easy-to-clean collecting plates. This reduces the risk of harmful particles in the air and eliminates the need to replace costly HEPA filters every month.
The Kronos® AIR 5G® Air Purifier destroys and eliminates dust, allergens, bacteria, and even viruses. The AIR 5G® has been third party lab tested and confirmed to kill 99.87% of influenza virus in one hour.
The patented system’s five step process starts with a pre-filter screen that filters and collects hair, pet dander, etc. The air is then pulled through emitter wires which create a 30,000-volt electro field that zaps dangerous particulates. In the ionic field, charged particles are destroyed, killing bacteria and pathogens. The particles are then captured on collecting plates, removing dangerous toxins from circulation. The collecting plate is easily cleaned and reused without buying new filters. The catalytic layer is the final step in the purification process, removing odors and keeping the air fresh and pure. The AIR 5G® has Smart Control Auto Mode, which measures and displays the air quality in the room and self-adjusts fan speed based on how dirty the air is in the room. There’s also an AIR 5G® Smart App that displays the real time Air Quality Index and acts as a remote control.
The Kronos® AIR 5G® Air Purifier is offered in three models:
- Kronos® AIR 5G® X3 air purifier combines powerful patented TPA® technology with a compact form factor up to six times smaller than other air purifiers, with washable and reusable filters.
- Kronos® AIR 5G® X5 thoroughly wipes out dust, smoke, dander, bacteria, pollen, viruses, odors, germs, and more from the air, delivering the healthiest breathable air possible. It was developed for some of the world’s most polluted areas and is now available for use in the home. It runs completely silently, passing through five stages of purification to guarantee the cleanest possible air in homes or offices.
- Kronos® AIR 5G® X8 delivers maximum power, more than doubling the capacity and efficiency of the Kronos X5, with CADR speeds of up to 470 CFM – enough to clean a 1,000ft2 room in just 20 minutes.
Kronos also offers the Kronos Car Air Purifier, the most advanced car air purifier with Kronos’ patented TPA® technology, and FitAir, the best personal air purifying solution that brings clean air anywhere by cleaning within 25ft2 of personal space at an airflow rate of 3x per hour.
Market Overview
The global air purifier market was valued at $10.38 billion in 2020 and is expected to reach $21.15 billion by 2027, achieving a CAGR of 10.7% over the forecast period, according to Brandessence Market Research. The market is primarily driven by the increasing concerns about both outdoor and indoor air pollution, coupled with the associated health problems.
Air pollution is one of the most prevalent concerns, due to worsening environmental condition. According to Health Effect Institute, it accounts for 4.9 million to 8.8 million deaths worldwide each year. Furthermore, as most of our time is spent is indoors, indoor air pollution remains a serious concern to individuals, as well as regulatory agencies. Particles like PM 2.5 can enter indoors through a wide range of sources including car engines, fireplaces, and coal- or natural gas and the infiltration of ambient particulates in urban areas. Even in the absence of solid fuels, indoor ventilation can build up PM 2.5 particles to a greater extent than in outdoor environments. Growing demand for portable air purification filters and systems in urban areas, increased advancements to catch key particulates like coronavirus, and increased regulatory measures to ensure safe environments for professionals in the industrial sector remain leading drivers of growth in the air purifier market.
Poor indoor air quality can cause fatigue, headache, and irritation of the eyes, throat, lungs, and nose, which can have a negative impact on worker productivity. Some air contaminants can cause asthma and other respiratory diseases.
Air purifier adoption is increasing rapidly in the U.S. to minimize health issues caused by poor air quality. Strict air quality standards, guidelines, and regulations in the U.S. are expected to have a positive impact on the market. For instance, the New Jersey Indoor Air Quality standard, NJAC 12:100-13 (2007), sets guidelines and standards related to indoor air quality during working hours in public employee-occupied buildings.
Key manufacturers are focusing on acquisitions and mergers to expand their geographical reach and strengthen their position in the market.
Management Team
Michael Rubinov, President and Head of Business Development
A seasoned hi-tech executive with 25 years of global business experience, Mr. Rubinov has served in various positions in sales, marketing, channel development and partner management. He has worked for large and global organizations such as Intel, NICE Systems, and Boeing (Defense and Security), as well as for start-up companies like Dialogic and Remunera International SA. He was appointed President and Head of Business Development of Kronos Advanced Technologies Inc. in February 2020. Mr. Rubinov holds an MBA, an MS Computer Sciences, and a BS Electrical Engineering.
Joseph L. Florence, Chief Operational Officer & CTO
A dynamic skilled leader in all aspects of business formation, evaluation, and execution, Mr. Florence brings a unique combination of Fortune 100 company experience with a lifetime of entrepreneurial experience to the Kronos team. He is a gifted visionary, possessing the unique ability to see future opportunities and make timely strategic adjustments and is naturally gifted at seeing unrecognized risk and overlooked opportunities. Mr. Florence has a proven track record of transforming companies to better align people, processes, and technologies to meet strategic goals and business metrics resulting in increased market share and profitability.
Laredo Oil Inc. (OTC: LRDC)
Laredo Oil Inc. (OTC: LRDC) is a publicly traded oil and gas exploration and production (E&P) company engaging in the acquisition and development of both undervalued quality conventional oil and gas properties and select mature oil fields that are suitable for the company’s proprietary Enhanced Oil Recovery (EOR) methods.
Laredo Oil is headquartered in Austin, Texas.
Conventional Acreage
Laredo Oil’s primary focus is on acquiring, developing, and operating undervalued conventional oil and gas properties.
The company leased 23,739 mineral acres in the Western Williston Basin of Montana, at favorable prices during the most recent down cycle and continues to take leases in the area. Before year end, it expects to drill the first development well at one of the first of 10 potential locations it has identified. If that well yields the anticipated results, the company plans to begin drilling additional wells there as soon as practical thereafter. The company believes the leased acreage has the potential to yield at least five years of development opportunities.
The company intends to pursue aggressively the acquisition of quality assets that major, mid-major, and large independent oil and gas companies continue to divest themselves of at a discount in response to ESG (Environmental, Social and Governmental) & sustainability initiatives and other pressures imposed upon them by their activist boards of directors. The company will focus on value, growth potential and free cash flow while complying with common sense ESG policies, often having a lower environmental impact than its competitors through its EOR methods.
EOR
In addition to pursuing conventional acreage and properties, Laredo Oil plans to acquire additional select mature oil fields where it believes that it can profitably use its proprietary Underground Gravity Drainage™ (UGD) model to recover stranded oil reserves (reserves previously considered to be economically incapable of recovery). The UGD method is applicable to mature oil fields that have very specific geological and reservoir characteristics.
Laredo Oil has done extensive research and field level application over the last 10 years and has identified specific oil fields within the United States that it believes are qualified for the UGD recovery method. The company believes the costs of implementing the UGD method are significantly lower than those of other commonly used EOR methods. Laredo Oil believes that it can materially increase the field oil production rate from prior periods and, in some cases, recover amounts of oil equal to or greater than amounts previously recovered from the mature fields selected.
Market Outlook
The company expects U.S. oil prices to climb in the near term as energy demand intensifies with the economy continuing to recover from the COVID-19 slowdown. Also causing upward price pressure is global supply chain dysfunction that slows or prevents shipments, including energy components, from reaching destinations. Domestic oil production is also constrained by years of reduced investment in fossil fuel producers due to green energy mandates. Accordingly, the company believes that the short-term outlook for oil is favorable. Many industries have yet to reach their pre-COVID production levels, which the company believes points to a continuing near-term upward trend in energy demand.
Management Team
Mark See has been the Chief Executive Officer and Chairman of the Board of Directors of the company since October 16, 2009. He has over 30 years’ experience in heavy civil, natural resources and the E&P industries. He was the founder and founding CEO of Rock Well Petroleum, a private oil & gas company until December 2008 and worked from then until October 2009 forming Laredo Oil. He was employed with Albian Sands as the Manager for the Alberta Oil Sands Projects at Fort McMurray, Alberta, Canada, a joint venture between Shell Canada and Chevron. Mr. See was also President of Oil Recovery Enhancement LLC in Bozeman, Montana, a private oil company. He was selected as one of the top 25 Engineers in North America by the Engineering News Record for his innovations in the petroleum industry. He is a graduate of the Mackay School of Mines at the University of Nevada at Reno, with a degree in Mining Engineering. He is a member of the Society of Mining Engineers and the Society of Petroleum Engineers.
Bradley Sparks currently serves as the Chief Financial Officer and Treasurer of Laredo Oil and has been a director of the company since March 1, 2011. Before joining Laredo Oil in October 2009, he was the Chief Executive Officer, President and a Director of Visualant Inc. Prior to joining Visualant, he was the Chief Financial Officer of WatchGuard Technologies Inc. from 2005-2006. Before joining WatchGuard, he was the founder and managing director of Sunburst Growth Ventures LLC, a private investment firm specializing in emerging-growth companies. Previously, he founded Pointer Communications and served as Chief Financial Officer for several telecommunications and internet companies, including eSpire Communications Inc., Digex Inc., Omnipoint Corporation, and WAM!NET. He also served as Vice President and Treasurer of MCI Communications from 1988-1993 and as Vice President and Controller from 1993-1995. Before his tenure at MCI, Mr. Sparks held various financial management positions at Ryder System Inc. He currently serves on the Board of Directors of Comrise. Mr. Sparks graduated from the United States Military Academy at West Point in 1969 and is a former Army Captain in the Signal Corps. He has a Master of Science in Management from the Sloan School of Management at the Massachusetts Institute of Technology and is a licensed CPA in Florida.
Donald Beckham has served as a director of the company since March 1, 2011. Since July 2015, he has been a partner with Copestone Energy Partners LLC. In 1993, he founded Beckham Resources Inc. (“BRI”), which, for over 30 years, has been a licensed, bonded and insured operator in good standing with the Railroad Commission of Texas. Through BRI, Mr. Beckham has drilled and operated fields for his own account. His expertise is in the acquisition, exploitation, exploration and production enhancement of mature oil and gas fields through which he has been able to enhance production by compressor optimization, pump design, work-over programs, stimulation techniques and identifying new pay zones. Prior to BRI, Mr. Beckham was the chief operations manager for Houston Oil Fields Corporation (“HOFCO”), where he began his career. There, he was responsible for drilling, production and field operations and managed approximately 100 people, including engineers, geologists, land men, pumpers, and other contract personnel, as well as state and federal environmental and regulatory functions. He managed an annual capital budget of approximately $30 million and operated approximately 100 wells. HOFCO drilled about 20 wells per annum and performed approximately 30 recompletions and work over operations each year. HOFCO owned interests in about 10 key fields principally in Texas, and company-managed production was approximately 1,000 bpd of crude oil and 10 mm cfd of natural gas. Mr. Beckham is a petroleum engineer and 1984 graduate of Mississippi State University.
Michael Price, an independent director of Laredo Oil, has over 40 years of senior financial and petroleum experience in the global oil and gas industry. He has been a principal in Octagon Energy Advisors, a Houston-based energy investment advisory firm, from 2002 to the present. The firm advises financial institutions and institutional investors participating in energy investments. From 2008 through his retirement in 2021, he was a Managing Director at ING Capital, which provides debt financing to domestic exploration and production companies. From 1998 through 2002, Mr. Price was the Chief Financial Officer of Forman Petroleum Corporation. Before that, Mr. Price was Managing Director at Chase Manhattan Bank for 15 years and was in charge of technical support for Chase’s worldwide energy merchant banking activities. In his early career, he worked as a consulting principal on domestic petroleum engineering and landowner matters and gained extensive international experience working with major oil companies in a variety of operating positions. He holds a BS and MS from Illinois Institute of Technology, an MBA from the University of Chicago, a M.Sc. from the London School of Economics, and an MS in Petroleum Engineering from Tulane University.
FORWARD-LOOKING STATEMENTS
This press release and the statements made by Laredo Oil, Inc. in this press release may be forward-looking in nature and are made pursuant to the safe harbor provisions of the Private Securities Litigation Reform Act of 1995. Such forward-looking statements describe Laredo Oil’s future plans, projections, strategies and expectations, and may be identified by words such as “expects”, “anticipates”, “intends”, “plans”, “believes”, “seeks”, “estimates” or the negative versions of those words or other words of similar meaning. These forward-looking statements are based on assumptions and involve a number of risks, uncertainties, situations and other factors that may cause the actual results, level of activity, performance or achievements of Laredo Oil or the oil industry to be materially different from any future results, level of activity, performance or achievements expressed or implied by these statements. These factors include changes in interest rates, market competition, changes in the local and national economies, and various other factors detailed from time to time in the reports filed with, or furnished to, the U.S. Securities and Exchange Commission, including its Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and Current Reports on Form 8-K. Laredo Oil undertakes no obligation to update publicly any forward-looking statements to reflect new information, events or circumstances after the date hereof to reflect the occurrence of unanticipated events.
Lexaria Bioscience Corp. (NASDAQ: LEXX)
Lexaria Bioscience Corp. (NASDAQ: LEXX) is a global innovator in drug delivery platforms. The company’s patented technology, DehydraTECH™, improves the way active pharmaceutical ingredients (APIs) enter the bloodstream by promoting healthier oral ingestion methods and increasing the effectiveness of fat-soluble active molecules. DehydraTECH promotes fast-acting, less expensive and more effective oral drug delivery and has been thoroughly evaluated through in vivo, in vitro and human clinical testing.
DehydraTECH is covered by 21 issued and more than 50 pending patents in over 40 countries around the world. Lexaria’s first patent was issued by the U.S. Patent and Trademark Office in October 2016 (US 9,474,725 B1), providing 20 years of patent protection expiring June 2034. Multiple patents have been awarded since then and are expected in the future.
Lexaria has a collaborative research agreement with the National Research Council (NRC), the Canadian government’s premier research and technology organization. The company has filed for patent protection for specific delivery of nicotine, vitamins, NSAIDs, testosterone, estrogen, cannabinoids, terpenes, PDE5 inhibitors (with brand names like Viagra), tobacco and more.
Lexaria began developing DehydraTECH in 2014 and has since continued to strengthen and broaden the technology. The company has no plans to create or sell Lexaria-branded products containing controlled substances. Instead, Lexaria licenses its technology to other companies around the world to offer consumers the best possible performance across an array of ingestible product formats.
The company’s technology is best thought of as an additional layer that providers of consumer supplements, prescription and non-prescription drugs, nicotine and CBD products can utilize to improve the effectiveness of their own existing or planned new offerings. Lexaria has licensed DehydraTECH to multiple companies, including a world-leading tobacco producer for the research and development of smokeless, oral-based nicotine products, and for use in industries that produce cannabinoid beverages, edibles and oral products.
DehydraTECH is suitable for use with a wide range of product formats including pharmaceuticals, nutraceuticals, consumer packaged goods and over-the-counter capsules, pills, tablets and oral suspensions.
DehydraTECH Technology
Lexaria’s DehydraTECH is designed specifically for formulating and delivering lipophilic (fat-soluble) drugs and active ingredients. DehydraTECH increases their effectiveness and improves the way active pharmaceutical ingredients enter the bloodstream. The major benefits to a subject ingesting a DehydraTECH-enabled drug or consumer product can be summarized by the following:
- Speeds up delivery – the effects of the product are felt by the subject in just minutes.
- Increases bioavailability – the technology is much more effective at delivering a drug or product into the bloodstream.
- Increases brain absorption – animal testing suggests significant improvement in the quantity of drug delivered across the blood-brain barrier.
- Improves drug potency – more of the ingested product is made available to the body, so lower doses are required to achieve the desired effect.
- Reduces drug administration cost – lower doses mean lower overall drug costs.
- Masks unwanted taste – the technology eliminates or reduces the need for sweeteners.
Lexaria has demonstrated in animal studies a propensity for DehydraTECH technology to elevate the quantity of drug delivered across the blood-brain barrier by as much as 1,900 percent, initiating additional new patent applications and opening possibilities for improved drug delivery.
Since 2016, DehydraTECH has repeatedly demonstrated, with cannabinoids and nicotine, the ability to increase bio-absorption by up to five to 10 times, reduce time of onset from one to two hours to just minutes, and mask unwanted tastes. The technology is to be further evaluated for additional orally administered bioactive molecules, including antivirals, cannabinoids, vitamins, non-steroidal anti-inflammatory drugs (NSAIDs) and nicotine.
Market Outlook
Lexaria’s ongoing research and development efforts are mainly focused on development of product candidates across several key segments:
- Oral Cannabinoids – a market estimated to be worth $18.4 billion in 2021 and expected to reach $46.2 billion by 2025.
- Antivirals – an estimated $52.1 billion market in 2021 that’s expected to grow to $66.7 billion by 2025.
- Oral Mucosal Nicotine – smokeless tobacco products, a $13.6 billion market in 2018, is forecast to grow at 7.2 percent annually through 2025.
- Human Hormones – estrogen and testosterone replacement therapies represented a $21.9 billion market in 2019, with a forecast CAGR of 7.7 percent through 2027.
- Ibuprofen and Naproxen – NSAID sales totaled $15.6 billion globally in 2019 and are projected to reach $24.4 billion by 2027.
- Vitamin D3 – the global market size was $1.1 billion in 2021, growing at 7 percent per year and expected to reach $1.7 billion in 2026.
Management Team
Chris Bunka is Chairman and CEO of Lexaria Bioscience Corp. He is a serial entrepreneur who has been involved in several private and public companies since the late 1980s. He has extensive experience in the capital markets, corporate governance, mergers and acquisitions, as well as corporate finance. He is named as an inventor on multiple patent innovations.
John Docherty, M.Sc., is the President of Lexaria. He is a pharmacologist and toxicologist, and a specialist in the development of drug delivery technologies. He is the former president and COO of Helix BioPharma Corp. (TSX: HBP). He is named as an inventor on multiple issued and pending patents.
Greg Downey is Lexaria’s CFO. He has more than 35 years of diverse financial experience in the mining, oil and gas, manufacturing, and construction industries, and in the public sector. He served for eight years as CFO for several public companies and has provided business advisory and financial accounting services to many large organizations.
Gregg Smith is a strategic advisor to Lexaria. He is a founder and private investor with Evolution VC Partners. He is a member of the Sand Hill Angels and held previous investment banking roles with Cowen and Company and Bank of America Merrill Lynch.
Dr. Philip Ainslie serves as a scientific and medical advisor to Lexaria. He is co-director for the Centre for Heart, Lung and Vascular Health, Canada. He is also Research Chair in Cerebrovascular Physiology and Professor at the School of Health and Exercise Sciences, Faculty of Health and Social Development at the University of British Columbia.
Lottery.com Inc. (NASDAQ: LTRY)
Lottery.com Inc. (NASDAQ: LTRY) is a next generation platform where consumers can play the lottery online – in browser or via smartphone app. The platform offers users access to official lottery games sanctioned by their individual states and also provides lottery data to more than 400 digital publishers, including Google and Amazon Alexa.
Lottery.com was founded in 2015, launching at the LAUNCH festival and soon turning into a leader in the industry. With headquarters in Austin, Texas, the company is dedicated to helping advance the lottery industry into the digital age and works closely with state regulatory bodies to achieve this goal.
The company recently entered into a definitive agreement for a business combination with special purpose acquisition company Trident Acquisitions Corp. (NASDAQ: TDAC) (“Trident”), which will result in Lottery.com becoming a publicly listed company. Once the transaction is complete, the combined company will be trademarked as Lottery.com, with its common stock to remain listed on Nasdaq under ticker symbol ‘LTRY’.
Lottery.com Online Platform
The Lottery.com online platform works closely with state regulators, advancing the lottery into the digital age. With the online platform, the company offers enhanced regulatory capabilities by leveraging innovative blockchain technology and capturing the untapped market of digitally native players.
Players go online in a browser or through a mobile application to use the interface. The process includes:
- Players Choose a Game: Players can play officially state sanctioned multi-state games and other games offered in the states in which they live. Players can also find winning numbers, jackpot totals, draw dates and more for hundreds of other lottery games around the world.
- Players Pick Numbers: Players can play their lucky numbers or do a quick pick of randomized numbers in as simple as two taps. “Tap, Tap, Ticket!”
- A Safe and Secure Way to Play: Purchases for up to 50 tickets can be made at one time through the online interface. Lottery.com handles everything after purchase, letting users know when they win.
- Collect All Winnings: Consumers keep 100% of their winnings. All winnings stay in the Lottery.com balance for future ticket purchases, or a cashout can be requested. Company representatives contact winners who hit big jackpots, instructing them on the redemption process.
A Better Way to Play the Lottery
Lottery.com has an innovative e-commerce platform that is using blockchain to maintain an accurate ledger. From 2016 to 2020, Lottery.com grew gross revenue at a CAGR of 363%, and it forecasts gross revenue equal to approximately $71 million in 2021, $279 million in 2022, and $571 million in 2023.
Lottery.com is leveraging a successful playbook, with $398 billion in global lottery sales but only 6.7% online penetration. The large market opportunity is expected to shift to online transactions within the next decade.
The platform is currently available in 12 states across the United States, and the company plans to expand to 34 by the end of 2023. Global expansion is also on the horizon, with partnership plans in Turkey and Ukraine.
Key features that make the Lottery.com experience unique include:
- All the Games Users Love – For consumers who live in applicable LIVE states, Powerball and Mega Millions are available right from the mobile application.
- Convenience – Lottery.com makes playing the lottery on mobile devices easy. After setting up an account, users can begin playing in moments or set reminders to play when the jackpot is high.
- Easy Cashouts – Users can cash out winnings straight to a bank account, safely and securely, with no commissions.
The company is also gamifying charitable giving, fundamentally changing how nonprofits engage with donors and raise funds. WinTogether.org is a platform designed to offer charitable donation sweepstakes to incentivize donors to take action by offering large cash prizes and once-in-a-lifetime experiences.
Strong Advisory Board Presence
Lottery.com is expected to continue to gain support, leaning on the experience of its advisory board and notable investors from the venture capital, gaming and entertainment industries. These include:
- Jason Robins, CEO of DraftKings Inc. (NASDAQ: DKNG)
- Ben Narasin, Venture Partner of NEA
- Peter Diamandis, Chairman of XPRIZE Foundation
- Matthew Le Merle, Co-Founder and Managing Partner of Fifth Era and Keiretsu Capital
- Paraag Marathe, President of Enterprises and EVP of Football Operations for the San Francisco 49ers
- Jamie Gold, The Poker Philanthropist
Management Team
Tony DiMatteo is the Co-Founder and Chief Executive Officer of Lottery.com. He is a serial entrepreneur and highly sought-after industry speaker and thought leader. He has been featured in The Wall Street Journal, Forbes, VentureBeat, TechCrunch Inc. and more for his approach to entrepreneurship, the gaming industry and cryptocurrency.
Matt Clemenson is the Co-Founder and Chief Commercial Officer of Lottery.com. He is responsible for the company’s strategy. Mr. Clemenson was steeped in corporate and enterprise engineering processes at Hotwire and Expedia before going on to be CEO at LesConcierges, the world’s largest concierge company, which merged into John Paul and sold to Accor Hotels. Clemenson and DiMatteo have been partners for more than 10 years.
Ryan Dickinson is the company’s President and Chief Operating Officer. He has a diverse background in business, technology, product, design and sales, which has aided him in producing many successful outcomes throughout his career. Notably, as Senior Vice President of a SaaS company, Mr. Dickinson produced profitability from a negative $1.4 million division within the first year by reinventing the product offerings, streamlining processes and establishing a go-to-market strategy. Additionally, he produced three record breaking revenue years in a row for AccuWeather, the world’s largest weather provider, by increasing every KPI for all flagship properties by no less than 5%.
Luc Vanhal is the company’s Chief Financial Officer. He has served in C-level executive roles since the 1990s, including a nine-year tenure for The Walt Disney Company (NYSE: DIS) from 1990 to 1999. From 2001 to 2004, he managed the development of the World of Warcraft massively multiplayer game, which, by the end of 2020, still had over five million active subscribers. As the CFO of Lottery.com, Mr. Vanhal leads the company’s global finance organization, with treasury responsibility, accounting, analysis and financial planning.
LQwD FinTech Corp. (TSX.V: LQWD)
LQwD FinTech Corp. (TSX.V: LQWD) (OTCQB: LQWDF) is a financial technology company focused on creating enterprise-grade infrastructure to drive bitcoin adoption.
LQwD FinTech’s mission is to develop institutional-grade services that support the Lightning Network and drive improved functionality, transaction capability, user adoption and utility, and scaling of bitcoin. LQwD is also securing a substantial position in bitcoin as an operating asset and will use its holdings to establish nodes and payment channels on the Lightning Network.
The Lightning Network is a second-layer protocol, sitting above the bitcoin blockchain, intended to facilitate faster micro-transactions and lower fees on bitcoin transactions, thus allowing mass adoption of bitcoin.
LQwD expects the Lightning Network to eclipse the patchwork of legacy financial networks that are used to move value today. The company’s software will make migration from legacy networks onto the Lightning Network easy and seamless. By onboarding more financial service providers, LQwD intends to grow the value of the Lightning Network.
The company, formerly known as Interlapse Technologies Corp., is harnessing new payment rails built on top of the bitcoin blockchain that are capable of beyond visa-level transaction volumes and backed by bitcoin, the strongest and most well-known cryptocurrency. These new rails, enabled by the Bitcoin Lightning Network, open a vast opportunity and market segment for digital payments and financial services on a global scale. LQwD aims to leverage its position as a public company to enhance trust in its products and services, and leverage its shares as currency for acquisitions, roll-up and growth, as well as to attract and retain top industry talent.
Product
The Lightning Network is a solution to massively scale the use of bitcoin for microtransactions globally, dramatically improving upon fees, as well as providing instant settlement times. The Lightning Network has experienced explosive growth and is expected to continue with the trend as usage increases. Well-known companies, such as Twitter and Square, have expressed their enthusiasm to incorporate Lightning Network into their platforms. The Lightning Network is scalable, global, open, inclusive, permissionless and decentralized. It is made up of nodes connected via payment channels, and enables off-chain, instantaneous and cheap payments at scale.
Upon launch of LQwD’s Lightning Network platform-as-a-service, users will be able to leverage the Lightning Network infrastructure to send payments instantly, securely and inexpensively anywhere in the world. Companies and service providers will be able to conduct Lightning Network transactions in bitcoin by integrating LQwD’s infrastructure with their business or web property. Connected businesses will be able to easily deploy, monitor and manage LQwD’s Lightning Network nodes with no or low-level technical knowledge required. The company fully expects Lightning Network to be a force for global change and to become the monetary exchange network of the future.
The Lightning Network, which is already built, functioning and growing, will advance bitcoin from a store-of-value to a global monetary network through payment utility. The company expects the Lightning Network will propel the growing number of active blockchain wallets to new heights, by increasing bitcoin’s scalability and lowering its fees for users. For coming generations, everything from wealth to experiences will be acquired and transacted virtually, and LQwD sees the Lightning Network as an enabling technology that can bring bitcoin to hundreds of millions of new users across the globe.
Market Outlook
Forbes in August 2021 noted that “private investors are funding companies that are building the infrastructure that will support future growth of crypto and digital assets,” and called public companies building cryptocurrency infrastructure “the hottest part of the crypto market.” While the first wave of investor interest in crypto firms was directed at companies catering to retail investors, investors have now shifted their attention to infrastructure builders, like LQwD FinTech. Forbes did not put an estimated value on the crypto infrastructure market but pointed out that large-scale adoption of cryptocurrencies will only happen when infrastructure is in place to support it. The larger digital payments market, of which crypto payments are a small fraction, is growing at more than 14 percent annually and is forecast to hit $154 billion by 2025.
Management Team
Shone Anstey is co-founder, chairman and CEO at LQwD FinTech. He has 20 years of experience in building complex technologies and has acted as technology lead for an industrial bitcoin mine and bitcoin mining pool. He is a Certified Cryptocurrency Investigator, and an advisor to the British Columbia Securities Commission. He is also co-founder of BIGG Digital Assets (OTCQX: BBKCF) and took that company public in 2017.
Barry MacNeil is CFO at LQwD FinTech. He is a member of the Chartered Professional Accountants of British Columbia and has more than 30 years of management and accounting experience with public companies and in private practice. His previous positions include director of both public companies and nonprofits, as well as Chief Financial Officer and Corporate Controller.
Albert Szmigielski is co-founder and CTO at LQwD FinTech. He was formerly the Head of Research and Chief Blockchain Engineer at Blockchain Intelligence Group and VP Research at CipherTrace. He holds a B.Sc. in Computing Science from Simon Fraser University, and a Master of Science in Digital Currencies and Blockchain Technologies from the University of Nicosia, Cyprus.
Marijuana Company of America Inc. (OTC: MCOA)
Marijuana Company of America Inc. (OTC: MCOA) operates and invests in the cannabis sector directly. The company’s diverse operations include cDistro, one of the THC, hemp & CBD cannabis industries’ fastest growing distribution companies; hempsmart™, a premium CBD company; and VBF Brands Inc., a cannabis nursery cultivation facility in Salinas, California, that is a cultivator and distributor utilizing its own growing systems to produce desirable cannabis clones.
MCOA continues to grow its business while remaining fiscally conscious and further establishing itself in the legalized cannabis THC, hemp & CBD industries by offering unique exposure to the global cannabidiol sector. The company intends to continue to leverage its premium brand hemp-based products with investments in and collaboration with existing and new strategic partners.
Marijuana Company of America offers investors the opportunity to be at the forefront of innovation in the legal cannabis and industrial hemp industries.
During the summer of 2021, the U.S. witnessed the introduction of the most comprehensive cannabis reform ever proposed at the federal level, as well as ongoing state-level liberalization. The investments MCOA has made will position the company to drive the expected strongest revenue growth in the company’s history.
MCOA strives to develop a comprehensive selection of synergistic companies that provides consistent value to its shareholders. Furthermore, its vertically integrated business model provides companies and partners with the best opportunities for rapid growth. It is MCOA’s attention to detail in producing premium products and adhering to the best business practices that distinguish it among the leaders of cannabis products in the global marketplace.
MCOA is building a portfolio of investments and joint ventures that represent the highest integrity and professionalism in the legal cannabis and industrial hemp markets. MCOA is a model for entrepreneurs and businesses that share its common goals and philosophies of not only creating value for investors but also creating an environment for businesses to improve the quality of life of customers through sustainable alternatives to many products currently on the market.
Partnerships and Investments
MCOA has partnered with and invested in a portfolio of companies operating in the cannabis sector. These include:
Cannabis Global Inc.
Cannabis Global Inc. (OTC: CBGL) is an emerging force in the cannabis marketplace with growing product and intellectual property portfolios. CBGL is marketing and producing Comply Bag™, an innovative solution for cannabis storage, transport, and tracking, and is also the developer and marketer of the Hemp You Can Feel™ brand.
Eco Innovation Group Inc.
Eco Innovation Group Inc. (OTC: ECOX) works with inventors and other professionals to nurture and catalyze the most innovative and impactful products and services and deliver those innovations to market. ECOX is dedicated to developing and commercializing successful products.
MCOA’s investment supports Eco Innovation’s cutting-edge extraction technology. ECOX’s extraction processes utilize a proprietary formulation to extract valuable bioactive compounds from cannabidiol (CBD) combined with plant-based materials to create a fluid and cost-effective output.
Together, both companies are positioned to identify and accelerate the development of new varieties of hemp-based products and distribute them worldwide.
Natural Plant Extract
MCOA owns a direct investment interest in Natural Plant Extract (NPE), which operates a licensed cannabis manufacturing and distribution business in Lynwood, California. NPE holds a Type 7 California manufacturing and distribution license, allowing for cannabis product distribution anywhere in the State of California.
Wholly Owned Subsidiaries
hempsmart™
hempsmart™ is a CBD company focused on creating and promoting the most effective, best tasting, and highest quality products on the market.
In 2021, hempsmart expanded into the global marketplace and announced a rebrand that featured a fresh take on its packaging and a social media campaign to engage customers via Instagram, Twitter, TikTok, and more, which has now generated a new loyal group of followers.
hempsmart premier products include its Smart Drops (CBD Drops), Neuro Smart (Patented Brain Pills), and Smart Cream (Pain Cream) brands. These organic, plant-based products help to manage anxiety, pain and insomnia, without the inclusion of THC.
cDistro
cDistro distributes CBD brands, along with smoke and vape shop-related products, to wholesalers, c-stores, specialty retailers, and consumers in North America.
cDistro was chosen as one of the first to distribute Marley One, the first global functional mushroom brand, in collaboration with the Bob Marley Family.
The initial product offering will include a range of functional mushroom tinctures, including species such as cordyceps, lion’s mane, chaga, reishi and turkey tail, that offer a range of unique health and wellness benefits, from immunity and gut health to cognitive function and sleep enhancement.
VBF Brands Inc.
MCOA recently completed the acquisition of VBF Brands Inc., a fully licensed marijuana cultivator and distributor based in Salinas, California. VBF utilizes its own growing systems to produce desirable cannabis clones that are designed to assist growers by reducing uncertainty and enhancing the likelihood of a successful cultivation harvest. Cannabis clones carry the exact same genetic potential as their mother plants and have similar cannabinoid and terpene profiles when grown properly.
This subsidiary will immediately work toward increasing production at its Salinas facility, which also offers exponential growth potential with other nearby properties that MCOA has an option to participate in as part of the acquisition.
Market Outlook
Ongoing changes in U.S. state government policies toward cannabis are expected to cause demand for legal marijuana to surge. In addition, the number of indications for which medical marijuana is prescribed continues to increase. These factors are expected to rapidly boost legal sales of cannabis products. Furthermore, an anticipated federal legalization of medical marijuana in the U.S. will increase opportunities for this market.
According to a Grand View Research report, the global legal marijuana market was valued at $9.1 billion in 2020. Market size is forecast to grow at a CAGR of 26.7 percent from 2021 to 2028. That would put the market value at roughly $30 billion by 2025.
The report cites the growing number of countries that are legalizing cannabis as a driver for surging demand. It also points out the use of medical marijuana for various ailments is gaining momentum worldwide. Medical marijuana is prescribed for patients suffering from chronic illnesses such as Parkinson’s, cancer, Alzheimer’s and other neurological disorders. The demand for cannabis oil is also increasing rapidly, especially among countries with legalized medical marijuana.
Management Team
Jesus Quintero is the CEO and Chairman of MCOA. From January 2013 to September 2014, he was the Chief Financial Officer of Brazil Interactive Media Inc. Since 2011, he has served as a financial consultant to several multimillion-dollar businesses in South Florida. He has extensive experience in public company reporting and SEC/SOX compliance and held senior finance positions with Avnet Inc., Latin Node Inc., Globetel Communications Corp., and Telefonica of Spain. His prior experience also includes positions at Price Waterhouse and Deloitte & Touche. He holds a B.S. in Accounting from St. John’s University and is a certified public accountant.
MedSmart Group Inc. (OTC: MSGP)
MedSmart Group Inc. (OTC: MSGP) is an investment company. On July 27, 2021, MedSmart announced it has agreed to acquire Milanion Ltd., a leading developer of disruptive autonomous and robotic solutions. The company further announced its appointment of Davinder Dogra, CEO of Milanion, as its new president. Through this acquisition, MedSmart will move forward with an aggressive roadmap to carve a share of the exponential growth in autonomous and robotic electric vehicle technologies utilizing artificial intelligence (AI) innovations.
Milanion Ltd.
Milanion Ltd. is an integrated defense and security technology company that designs and manufactures advanced defense equipment and systems engineered to provide sophisticated capability solutions in the autonomous and robotic sector. By leveraging the latest AI technologies, the company creates effective, battle-ready systems designed for the most demanding missions, providing superior flexibility, scalability, and reliability in land, marine and air use cases.
Milanion’s strategy focuses on identifying and acquiring best-in-class technologies to enhance and expand its current offerings while advancing in-house development to expand its product range. These initiatives position the company to take advantage of the expanding defense and civilian markets for robotic and autonomous technology systems and solutions.
Milanion is also expanding into public and commercial sectors in markets around the world. Its current and potential commercial applications and markets include roads and infrastructure, oil and gas, power and utilities, security, fire and rescue, mining, and agriculture. Guided by a corporate culture of decentralization, Milanion works with end-user partners, utilizing its capabilities, authority, and flexibility to respond quickly to changing market conditions and deliver tailored solutions based on local preferences.
Milanion’s product portfolio has been developed by working in consultation with and gaining feedback from end-users to augment and transform team and mission capabilities to make informed decisions, increase safety and productivity, and expand reach and access. The platforms can be built from the ground up as bespoke solutions or as conversions of existing platforms for use in a range of public and commercial sectors.
The AGEMA UGV
Milanion’s AGEMA is a modular, multi-mission unmanned ground vehicle (UGV) designed to support infantry and special forces units operating in both mounted and dismounted roles. The AGEMA is a proven platform that has been tested to perform in a range of environments, including extreme desert temperatures of up to 50 degrees Celsius. With rugged construction and amphibious capabilities, it can operate in a variety of challenging off-road environments, including muddy swamps, river and streams, and dense jungle.
The flexible UGV can be programmed to follow other vehicles as part of a convoy, follow troops at a specified distance, or lead a group of soldiers as a reconnaissance and force projection capability. This versatility makes the AGEMA ideal for providing actionable intelligence and offensive capability while keeping personnel out of harm’s way.
With the ability to be equipped with a variety of payloads and technologies to support a wide array of mission profiles, the AGEMA UGV is suitable for a range of mission roles, including:
- Fire Support and Remote Weapons Stations – The AGEMA can be equipped with a variety of remotely controlled weapons systems, such as anti-tank missiles.
- Load Carrying – The powerful UGV can ease the burden of troops by carrying ammunition, rations, heavy weapons, radio equipment, and more.
- Communications Support – The AGEMA can aid in the deployment and recovery of remote communications systems and infrastructure.
- Medical Support – The vehicle can serve as a mini-mobile theater or perform casualty evacuation operations.
- Route Clearance – The AGEMA can be fitted with ground-penetrating radar, explosive and ordnance chemical detector sensors, and other equipment that allows IED and route clearance patrols to be conducted without endangering personnel.
- Surveillance and Monitoring – A wide variety of cameras and imaging payloads can be fitted to the AGEMA to aid in ISR, mapping, search and rescue, and other missions where enhanced situational awareness is of paramount importance.
- Drone Launch and Support – Fixed-wing drones can be skid-launched from the AGEMA, and the platform can also support persistent rotary drone operations via a tethered system.
This modularity uniquely positions the AGEMA to provide assistance across a number of mission support sectors, including:
- Defense
- Civil Defense
- Oil & Gas
- Prisons and Refugee Camps
USV Conversions
The unmanned surface vessel (USV) market is mature and expanding, with such vessels taking part in operations across the globe. Supporting a range of missions, USVs are used for port security, transatlantic and transpacific marine surveys, defense and security applications, river and stream conservation study and exploration for oil and gas.
Owing to changing regulation and rapidly advancing technology, Milanion has experienced a trend in recent times of clients favoring USV conversions rather than purpose built unmanned vessels. Milanion’s experience with USV conversions offers a number of benefits to its client base, such as:
- The ability to continue using existing assets without any compromising of the original manned operation capabilities
- Avoidance of additional maintenance training or required expansion of logistics and support, since the converted USVs maintain the same operating characteristics of the existing fleet.
- The option for clients to scale capabilities and upgrade equipment overtime by leveraging the “plug and play” functionality of Milanion’s operating system.
- Vehicle conversion can be completed in as little as 3-4 weeks, greatly outpacing the commissioning of new vessels while offering substantial cost savings.
With Milanion’s autonomous technology, almost any vessel can be converted into a USV. Users can remotely upload and manage mission plans and waypoints and control throttle and steering, as well as third-party sensors and systems.
Industry and Market Outlook
The global robotics and autonomous systems market of the defense sector is estimated to reach $26.13 billion by 2025, expanding at a CAGR of 20.75% during the forecast period from 2020 to 2025, according to data from BIS Research. The entire market for such products is forecast to be $66.9 billion, with the industrial sector accounting for the lion’s share, as detailed in MedSmart’s July 27, 2021, news release.
Milanion is focusing development to aggressively service that demand and will employ its technology to be a sector leader. Its strategy focuses on continued product development and pursuit of accretive acquisitions, coupled with an increased global sales footprint.
With a regimented mandate to deliver on promises made – on time and within budget – Milanion CEO Davinder Dogra leads an efficient and effective operation with a dynamic management team, bringing decades of industry experience in the global development and sales of defense systems. The company is focused on growing Milanion’s land, marine, and AI divisions and making inroads into the industrial and commercial sectors.
President
Davinder Dogra is the President and CEO of Milanion. He has decades of working knowledge, experience, and expertise building successful businesses from the ground up, servicing global defense sector markets in Asia, Africa, the Middle East, and Europe.
Mr. Dogra founded and became Chief Executive Officer of integrated defense and security company Milanion Group in late 2019. Under his vision, leadership, and strategic guidance, Milanion has evolved from humble beginnings to establishing a global presence and becoming a leader specializing in the design, development and manufacture of effective, affordable unmanned autonomous platforms and systems based on advanced artificial intelligence (AI) and robotics technology.
In a time of evolving security threats and global interdependence, Mr. Dogra has established himself as an indispensable asset with an unrivalled global pedigree based on practical, working knowledge and insight, with a focus on delivering operationally relevant capabilities and results-oriented solutions to global defense markets. He has become the primary resource to customers through strategic insight, a solution-oriented collaborative approach and a deep understanding of the needs and complexities of the industry on a local, national, and international level. Ultimately, his efforts are aimed at empowering government organizations and private entities to protect borders, communities, and lives.
Over the course of two decades, Mr. Dogra has built his presence and reputation in the global defense industry as a trustworthy, reliable, and forward-thinking asset, providing access to innovation, efficiencies, and agility. With a focus on delivering operationally relevant capabilities and results-oriented solutions to global markets, his business achievements include a partnership with Eastern Europe’s largest defense equipment manufacturing conglomerate.
Prior to working in the defense industry, Mr. Dogra spent over two decades building businesses from the ground up to become leaders in their field – most notably, a leading Corporate and Government Affairs company providing strategic business intelligence, insight and advocacy for organizations looking to enter the Indian and other emerging markets around the globe. The business achieved recognition as one of India’s top corporate and government affairs service providers, becoming the first port of call for Fortune 500 businesses, SMEs and foreign government departments looking to do business in India.
Mr. Dogra’s career achievement highlights include:
- Integral part of UK Department of Trade and Industry (DTI) delegations at the forefront of opening business ties with India at the start of India’s business liberalization phase.
- Senior Director at Albright Stonebridge Group, a leading Washington DC-based corporate & government affairs group headed by former U.S. Secretary of State Madeleine K. Albright, former National Security Advisor Samuel R. “Sandy” Berger and former Senator Warren Rudman.
- Accompanied U.S. President Clinton as part of a business delegation promoting stronger links between the U.S. and India.
- Original founding, board and charter member of international networking and business start-up organization launched in the UK – Tie UK.
- Founded and attained the premier position for a consultancy delivering solutions to defense and security related issues faced by international companies in India.
- Lead the successful domestic and international expansion as director of India’s leading coach, bus and components manufacturer.
- Established numerous successful international start-ups and joint ventures in a broad spectrum of industries, including defense, security, mineral resources and real estate.
Mind Cure Health Inc. (CSE: MCUR) (OTCQX: MCURF)
Mind Cure Health Inc. (CSE: MCUR) (OTCQX: MCURF) (“MINDCURE”) is a diversified life sciences company at the forefront of the mental health industry. The company is currently developing digital therapeutics and researching psychedelic compounds, while innovating and commercializing new ways to promote healing and improve mental health.
MINDCURE’s research and digital therapeutics technology supports access to safe, science-based, evidence-backed psychedelic-assisted therapies globally. With hundreds of millions of people suffering from mental illnesses worldwide and an estimated $1 trillion in lost productivity per year, psychedelics offer promising alternatives for healing. This medical need has been amplified by the COVID-19 pandemic. According to the Centers for Disease Control and Prevention, 40 percent of U.S. adults reported struggling with substance abuse or mental health issues during the pandemic.
MINDCURE is uniquely positioned to address these medical needs. By concentrating on both technology and research, the company is focusing on near-term revenue generation, targeting a longer-term, blue sky horizon and hedging against regulatory unknowns with a scalable, adaptive model. MINDCURE’s software-as-a-service (SaaS) platform, iSTRYM, scales globally and services every psychedelic medicine without the capital-intensive drag of clinic scale-out costs. The company plans to first enter the market for psychedelic-assisted psychotherapy, then to move into the larger fields of technologically undisrupted psychotherapy and psychiatry.
Technology
Digital therapeutics include health interventions delivered through a smart device to induce a behavioral change in the patient. The global market is focused on simplifying behavioral change and empowering consumers to take charge of their own health. iSTRYM is the company’s AI-driven software platform that enables personalized and quantified outcomes in psychedelic therapy. The SaaS platform modernizes care, taking it from manual to digital and bringing better treatment outcomes for patients and therapists while lowering costs for insurers.
iSTRYM offers clinicians direct access to global, science-backed, evidence-based protocols, integration plans, insights into client journeys, and real-time assessments for personalized care. Patients access the platform on their smart devices, enjoying transparency into their wellness journeys, personalized care resources, and optimized relationships with their practitioners. The minimal viable product (MVP) of the software is being launched in Q3 2021. MINDCURE targets a Q1-Q2 2022 commercial product launch.
Research
In June 2021, the company announced it had completed the first stage of manufacturing pharmaceutical-grade synthetic ibogaine to be used in clinical research. In July, MINDCURE announced it had filed U.S. Provisional Patent applications for the company’s first full synthetic routes to create ibogaine. The company’s pharmaceutical grade ibogaine would provide researchers access to a sustainable, high-quality, reliable, and consistent supply of the psychedelic drug.
The company is also actively researching ibogaine as a potential treatment for Traumatic Brain Injury and related conditions. Preliminary data show the drug may also have promise as a treatment for neuropathic pain and migraines. In addition, research indicates ibogaine may help repair and rewire the brain’s neural pathways, making it potentially useful in the treatment of addictions.
Market Outlook
MINDCURE actively develops technology, conducts research, and distributes products in several market spaces. The global market for digital therapeutics is projected to grow to $6.9 billion by 2025, from an estimated $2.1 billion in 2020. In North American alone, the market is forecast to reach $5 billion by 2025.
The market for treatment of drug, alcohol and other addictions is estimated to be worth $38.2 billion in 2021, with a forecast CAGR of 5.2 percent for the next several years. The global market for the treatment of neuropathic pain is forecast to account for $9 billion by 2027, while drug treatment for migraines is expected to have a value of $2.1 billion by 2025.
Management Team
Kelsey Ramsden is President and CEO of MINDCURE. She has 15 years of experience founding, scaling, and operating innovative companies across Canada and the Caribbean. She has built multiple eight-figure businesses and twice been named Canada’s Top Female Entrepreneur. She holds a seat on the Entrepreneurship Council for the University of Western Ontario, where she is also a faculty member. She has an MBA from the Richard Ivey School of Business at the University of Western Ontario.
Dr. Joel Raskin is the Chief Medical Officer at MINDCURE. He is a psychiatrist and academic with 20 years international pharmaceutical experience in neuroscience drug development, lifecycle preparation, launch and commercialization with Eli Lilly & Co., where, as Senior Director, he led the medical affairs team for Alzheimer’s disease diagnostics and therapeutics. He earned his medical degree from the University of Toronto and is a Fellow of the Royal College of Physicians and Surgeons of Canada in Psychiatry.
Tarik Lebbadi is the COO at MINDCURE. He has more than 13 years of international operational experience. Before joining the company, he led the medical division of Johnson & Johnson in Morocco. He holds a BA in mathematics and computer science from Ripon College and an MBA from IESE Business School in Barcelona, Spain.
Geoff Belair is the CTO at MINDCURE. He has 30 years of experience working in highly regulated industries, including fintech and banking. He was the senior architect and creator of the Integration Services Team at banking solutions company Fincentric Corporation. Before joining MINDCURE he was Vice President of Information Technology at Westland Insurance.
Michael Wolfe, CPA CA, is MINDCURE’s CFO. He has 30 years of experience in finance, accounting, private equity, and business valuation. He was previously CFO of Baylin Technologies Inc., as well as CFO of several mid-market Canadian companies, including Masstech Group Inc. He was General Partner at VenGrowth Capital Partners Inc. He holds an MBA from McMaster University and a BA in business and economics from the University of Western Ontario.
Daniel Herrera is Vice President of Growth & Strategic Partnerships at MINDCURE. He is a former pharmaceutical executive with extensive experience in highly regulated industries. He is experienced with medical affairs, product development and product licensing, negotiations with public and private payers, GPOs, and pharmacy buyers, as well as strategic partnerships resulting in high-value M&A transactions. He is a graduate of McGill University and the University of Montreal and holds an MBA from the John Molson School of Business at Concordia University.
Mobius Interactive Ltd.

Mobius Interactive Ltd. is an online gaming operator launching in September 2020 with a variety of unique offerings catering to diverse demographic groups. In partnership with Ultra Play, a leading eSports and iGaming platform, Mobius Interactive is seeking to attract a network of high-net-worth gamers from around the world through the use of loyalty and gamification programs designed to enhance customer engagement by leveraging state-of-the-art customer relationship management systems and joint-ventures with over 600 VIP and Master gaming affiliates.
Array of Brands
Mobius Interactive is seeking to target a variety of customer segments and geographies through its diverse brand offerings, including:
- Aragon Casino: Austria, Finland, the Balkans, Canada, Africa and New Zealand
Catering to consumers aged 21 to 45, Aragon Casino brands itself along the lines of medieval fantasy, mimicking elements from the likes of The Walking Dead and Game of Thrones. - Club Double: Austria, India, Brazil, Finland, Canada, Africa and New Zealand
Targeting the 30 to 65 age demographic, Club Double is designed to exude a classic yet magical old Hollywood and vintage Miami & Las Vegas air. - MobiusBet: Germany, Austria, Switzerland, Brazil, Latin America, New Zealand and India
MobiusBet is designed to appeal to the 18- to 38-year-old eSports community, bringing together loyalty programs, targeted gamification and product merchandising in one seamless package.
Key Differentiating Indicators
Mobius Interactive has designed its platform with a number of key differentiation traits relative to its target market. These include:
- The use of affiliates: Mobius Interactive has partnered with over 600 VIP and Master gaming affiliates, who will introduce high-value players to the company’s award-winning iGaming platform. Mobius added over 150 proven affiliates in Europe, Brazil, Finland and New Zealand over a period of just 20 days.
- eSports Focus: Mobius.Bet, Mobius Interactive’s dedicated eSports hub, will cater to the quickly growing eSports segment, which is expected to rise to a value of $1.7 billion in 2021. With Mobius’ COO being one of the original founders of the eSports.com brand, the company aims to capitalize on this growing segment of the gaming industry.
- Customer Relationship Management (CRM): Mobius has partnered with Solitics, a new and real-time CRM system, enabling the company to personalize customers’ gaming experiences in an interactive and highly intelligent manner.
- Loyalty & Gamification: Mobius Interactive is set to introduce a unique loyalty and gamification program designed to increase customer engagement from signup. Loyalty and gamification programs have been proven to increase daily active wagering volumes by 30% while simultaneously increasing daily player activity by 60%. Furthermore, the introduction of these programs can help lower the company’s customer acquisition costs while adding a differentiating element to its platform.
Partnership with Puurl
Puurl provides a solution that embeds eGaming platforms into any existing online e-commerce store. First, shoppers can install the Puurl add-on to their browsers. Then, when visiting their preferred e-commerce stores, players will be prompted to bet, with the potential to win the products they’re browsing. The Puurl solution enables e-commerce operators and eGaming platforms to earn additional gambling revenues – even when their players are shopping. Through its partnership with Puurl, Mobius Interactive will look to add a unique revenue stream to complement its core business operations.
Management Team
Lynn Pearce, CEO, is an experienced, data-driven, commercially focused, strategic brand marketer with over 15 years of proven success in the global gaming industry, from land-based casinos in the UK to online gaming companies offering sports betting, poker and casino games. She was head-hunted to join a startup in Prague that launched 26 casinos, becoming profitable within the first three months of operation, before she relocated to Malta to join a leading B2B casino software development company as head of marketing, where she led global marketing, PR, product development, branding and go-to-market campaigns, retaining full control of a six-month budget of €1 million to increase brand awareness and customer engagement. She recently returned to the B2C side of gaming to launch three new brands in Germany, Brazil and India. She writes articles regularly for Infinity Gaming Magazine and has been a judge for the prestigious International Gaming Awards, a significant event for the gaming industry held each year prior to the largest gaming exhibition of the year, ICE London.
Robin Lawson, Vice President & COO, has been involved in iGaming for over 10 years, successfully founding two VIP casino departments across international locations in Latin America, as well as startup company Tabella in Europe. He most recently co-founded and acted as COO for eSports.com, which raised over $5.5 million as a startup ICO and was sold to German media giant ProSieben. Lawson is also a senior iGaming consultant for startup casino groups and an advisor to blockchain-based tech groups. His long-time experience and proven track record in startup organizations demonstrate his operational leadership skills.
Nicholas de Freitas, Vice President, Marketing, is one of the pioneers of digital stills photography for major retail companies in Africa and Australia. He left to start up UrbanActive, an outsourcing agency, working as marketing project manager and implementing major retail projects. He received his certification in digital marketing from the University of Stellenbosch. He has worked over the past few years as the marketing manager for various poker rooms and casinos, liaising and building relationships with software developers, successfully implementing a number of casino and poker products and holding regular weekly report sessions with the heads of all divisions of the company, spanning South Africa, Canada, Malta, Norway and Costa Rica.
Gary Eldridge, Chairman, is an experienced entrepreneur with a history of working in the venture capital and private equity industry. He is skilled in capital markets, M&A and funding startups and is a strong business development professional. For the past 30 years, he has created and managed numerous public and private companies in Canada, the U.S., Amsterdam, London, Zurich, Dusseldorf, Singapore and Panama. In addition to holding the role of chairman of the company, Eldridge is acting as a mentor to the team, assisting with the financials and structure of the company while allowing the team to be fully focused on Mobius’ growth and operations.
Mydecine Innovations Group Inc. (NEO: MYCO) (OTC: MYCOF)
Mydecine Innovations Group Inc. (NEO: MYCO) (OTC: MYCOF) is a biotechnology and digital technology company aiming to transform the treatment of mental health disorders and addiction. Founded in 2020 on the guiding principle that there is a significant unmet need and lack of innovations in the mental health and therapeutic treatment environments, Mydecine is dedicated to efficiently developing innovative first- and second-generation novel therapeutics to treat PTSD, addiction and other mental health disorders.
Mydecine’s business model combines clinical trials and data outcome, technology and scientific and regulatory expertise with a focus on psychedelic therapy underpinned by novel molecules with differentiated therapeutic potential. By collaborating with some of the world’s foremost authorities connected by best practices, Mydecine aims to responsibly fast-track the development of new medicines across its platforms, ultimately changing the way we treat mental health disorders. The company seeks to bridge the gap between the needs of patients and what the mental health care system currently provides.
Mydecine Innovations Group is headquartered in Denver with international offices in Canada and Europe.
Research and Technology
The invention and development of novel psychedelic and non-psychedelic molecules for medical use is an important part of Mydecine’s research strategy. The company uses molecules found in nature as building blocks to create improved second-generation drugs. This portfolio of new drugs represents major improvements to existing natural products and synthetics, including enhanced safety, efficacy, stability and dosing, as well as reduced side effects.
The goal of creating these improved second-generation compounds is to enable safer, more effective treatments for patients, along with improved management of dosage and drug behavior for clinicians. Mydecine believes the multibillion-dollar market for mental health and addiction disorder medicines will soon be disrupted amid a resurgence of the study into psychedelics and data showing the immense benefits of these forms of medicine.
The company currently has four lead drug candidates which include various enhancements such as improved controllability, delivery mechanisms, safety, stability and shelf-life. The drug candidates are in clinical trials or in pre-trial stage as potential treatments to aid PTSD, substance abuse and smoking cessation.
Mindleap Health is a wholly owned subsidiary of Mydecine. The Mindleap platform is a virtual community that aims to foster the conscious and responsible adoption of psychedelic medicine into inner wellness. Users access the platform through the Mindleap app. Mindleap provides users with inner wellness resources to assist them in their daily mental-health journeys. The platform also seeks to support the conscious and trustworthy adoption of psychedelics into a widely accepted approach to mental health and inner wellness.
Market Outlook
The global smoking cessation market is expected to reach $63.99 billion by 2026, growing at a CAGR of 16.9 percent from 2018 to 2026. The market for psychedelic therapeutics is in its very early stages. Estimates of current market value and forecasts of expected value in future years are all over the map. Market forecasts range from $6.5 billion by 2030 with a CAGR of 15 percent, to more than $69 billion as soon as 2025, at a CAGR of 8.2 percent. What is clear is that interest in psychedelic therapeutic drugs is expanding rapidly.
Management Team
Joshua Bartch is Chief Executive Officer and Chairman of Mydecine Innovations Group. He is an experienced entrepreneur who co-founded AudioTranscriptionist.com and founded Denver-based dispensary Doctors Orders in 2009. He also founded a boutique investment firm that operated throughout the U.S. and Canadian markets. In 2014, Bartch co-founded Cannabase.io, the USA’s most significant and sophisticated legal cannabis wholesale platform.
Dr. Rakesh Jetly, OMM, CD, MD, FRCPC, is the Chief Medical Officer of Mydecine. He was formerly Chief of Psychiatry for the Canadian Armed Forces, retiring in 2021 with the rank of colonel after 31 years of service. He began his career as a general duty medical officer and flight surgeon and spent his final 20 years of service as a psychiatrist. He maintains academic appointments at Dalhousie University and The University of Ottawa. He is the inaugural CF Brigadier Jonathan C. Meakins CBE, RCMAC, Chair in Military Mental Health at the Royal Ottawa Hospital.
Robert Roscow is Chief Scientific Officer of Mydecine. As a geneticist, he has spent his academic and professional careers looking for valuable and unique medicinal molecules found in nature. His innovations were applied at Canopy Growth and ebbu, where he ran those companies’ genetics divisions. He has leveraged his expertise to maximize industrial production of cannabinoids in a pharmacological context, resulting in multiple patent filings.
Damon Michaels is Chief Operating Officer of Mydecine. He previously consulted for various hemp businesses through his company, Emerald Baron. Before that, he served as GM for ebbu, the leading multi-platform cannabinoid research and technology firm based in Colorado. He has held leading roles with multiple large brands throughout the cannabis vertical. He also developed a national snowboard brand.
Nemaura Medical Inc. (NASDAQ: NMRD)
Nemaura Medical Inc. (NASDAQ: NMRD) is a medical technology company developing affordable diagnostic and digital tools for chronic disease management. Its flagship product, sugarBEAT®, is a wearable, non-invasive and flexible Continuous Glucose Monitor (CGM) designed to help people with diabetes and prediabetes manage their glucose levels. Insulin users can adjunctively use sugarBEAT when calibrated with a finger-stick glucose reading.
sugarBEAT consists of a daily disposable adhesive skin patch connected to a rechargeable transmitter with a smartphone app displaying glucose readings at five-minute intervals for periods of up to 24 hours. One of the great advantages of the product, apart from the fact that users no longer need to draw blood samples or prick their fingers, is that a person can wear the CGM patch on whatever day they choose. Existing CGM devices must be implanted under the skin. Wearable disposability is a unique feature of sugarBEAT and a world first, opening up vast potential for changing the way people manage their chronic disease conditions. sugarBEAT received CE mark clearance in May 2019, allowing it to be marketed and sold within the European Union as a Class 2b Medical Device. The company submitted a premarket approval (PMA) application to the U.S. Food and Drug Administration in 2020 which is currently under review.
Founded in 2011, Nemaura set out to develop a single platform technology to measure blood markers at the surface of the skin. Since then, the company has evolved with the creation of wearable technologies and digital health care solutions that encourage and empower people to take charge of their own health and well-being. Nemaura’s skin surface blood monitoring technology has allowed the company to create additional products, which are in the pipeline, such as Lactate Monitoring.
Technologies
Digital Solutions for Weight Loss and Potential Reversal of Type 2 Diabetes
This is a digital program that comes with more than a decade of clinical evidence demonstrating excellent efficacy. The company has combined this with its glucose-monitoring platform to bring a product to market to help people with diabetes manage their condition and potentially reverse Type 2 diabetes.
Glucose Monitoring Solutions for Diabetes Prevention and Reversal
Over 420 million people worldwide are living with diabetes, and prediabetes cases total almost three times that number. Undoubtedly, diabetes is an urgent global health crisis. Combining clinical research with patient-friendly technology, Nemaura’s sugarBEAT product delivers a non-invasive, affordable and flexible method of blood glucose tracking for improved diabetes management.
Continuous Lactate Monitoring for Athletic Performance (Non-Medical)
Lactic acid is a key performance indicator for the body and a guide to how well muscles react to long term exertion and recovery. Well-trained athletes and those who regularly engage in sports are very efficient at faster lactate ‘recycling’ for extra energy (ATP). Nemaura expects to launch its lactate sensor to the sports and personal training market in 2022.
Continuous Lactate Monitoring in Disease State (Medical)
An increase in blood lactate levels is also a marker of critical disease states. Recent publications have indicated the presence of elevated lactate levels in patients with COVID-19 infection. Nemaura has developed a lactate sensor that is being integrated into the company’s platform, which will be submitted for regulatory clearance upon completion of requisite clinical studies.
Continuous Temperature Monitoring for Viral Infection Detection and Disease Progression
A person’s body temperature says a lot about their health. Several diseases, including COVID-19, are characterized by an increase in body temperature, so temperature monitoring is a vital tool in the detection, diagnosis and prevention of the spread of disease. Nemaura is expecting to submit this adaptation of the device for regulatory clearance in 2022.
Market Opportunity
Obesity and diabetes are two of the major drivers of the current chronic disease epidemic. According to the International Diabetes Federation, there are more than 463 million people living with diabetes worldwide. In the U.S., about 28,000 people are diagnosed with diabetes every week, and more than 34 million suffer from diabetes. Another 88 million Americans have prediabetes. Other industrialized countries show similar numbers based on their populations. In the U.K., 4.8 million people have diabetes, with another diagnosed every two minutes. In Germany, 9.5 million have diabetes, with almost half estimated to be undiagnosed and so at greater risk.
On average, employers and insurers spend more than $9,000 annually on health care for an employee with diabetes, compared to $1,600 annually for a healthy employee. In the U.S. alone, more than $760 billion was spent on diabetes-related health care expenditures during 2019. Nemaura is positioned at the intersection of the global Type 2 diabetes market that is expected to reach nearly $59 billion by 2025, the $50-plus billion prediabetic market, and the wearable health-tech sector for weight loss and wellness applications forecast to hit $60 billion by 2023.
Management Team
Dr. Faz Chowdhury has been CEO and chairman of the board of Nemaura Medical since 2013. He has more than 20 years of experience in the pharmaceutical and medical devices industry, taking products from concept to commercial launch. He is sole inventor on more than 100 granted and pending patents and has authored textbook chapters on nano-biosciences for Wiley and Elsevier. He holds a master’s degree in microsystems and nanotechnology from Cranfield University, and a doctorate from the University of Oxford in nano-medicine and drug delivery.
Justin Mclarney is CFO at Nemaura. He most recently was the Senior Director, International Finance at Lands’ End Inc. He also worked for Office Depot as Senior Director of Finance for the largest business unit within the European group. Prior to that, he spent more than 10 years in practice, the majority of which was with Ernst & Young LLP.
Dr. Fred Schaebsdau is Vice President of Strategy & Strategic Alliances at Nemaura. He has more than 15 years of executive experience in the CGM, blood glucose monitoring and insulin delivery industries, including time with Abbott Diabetes Care, as General Manager of Dexcom Germany and at Roche Diabetes Care, where he was Senior Vice-President, Head of Global Strategy and Business Development. The firm he founded is the exclusive distributor in Europe, the Middle East and Africa of UniStrip®, the world’s first generic blood glucose test strip. He is licensed to practice medicine in the U.S. and Germany.
David Scott is Director of Commercial Development and Licensing at Nemaura. He is a trained chemist with over 35 years of experience in the pharmaceutical industry, including deal brokering, marketing, strategic planning, finance, business development and acquisitions. He has also provided licensing training for a number of multinational pharma companies and training organizations and is the author of best-selling report Scrip’s Practical Guide to Pharmaceutical Licensing.
Mullen Automotive Inc. (NASDAQ: MULN)
Mullen Automotive Inc. (NASDAQ: MULN) is a Southern California-based automotive company that owns and partners with several synergistic businesses working toward the unified goal of creating clean and scalable energy solutions. Mullen has evolved over the past decade in sync with consumers and technology trends. Today, the company is working diligently to provide exciting EV options built entirely in the United States and made to fit perfectly into the American consumer’s life. Mullen strives to make EVs more accessible than ever by building an end-to-end ecosystem that takes care of all aspects of EV ownership.
Commencement of Trading on Nasdaq
On November 5, 2021, Mullen announced its commencement of trading on the Nasdaq Capital Market.
“Today is a monumental day for Mullen Automotive. I am especially proud of our team, investors and all who have believed in Mullen and taken us to this point as a publicly traded company on the Nasdaq Capital Market,” David Michery, CEO and Chairman of Mullen Automotive, stated in the news release. “Trading on Nasdaq now opens us up to new investors, both institutional and retail shareholders, and broadens our awareness and company profile, while increasing awareness of Mullen and our technology platform and opening new opportunities in EV and beyond. The road ahead has never been brighter for Mullen, and I am proud to lead us into the future.”
The milestone came in the wake of the company’s stock-for-stock merger with Net Element Inc.
The Mullen FIVE
The Mullen FIVE EV Crossover, debuting at the Los Angeles International Auto Show (LAIAS) on November 17, 2021, embodies Mullen’s Southern California roots with an inspired design focused on two complementary Golden State themes – California landscape and California urban.
The FIVE is built on an EV Crossover skateboard platform that offers multiple powertrain configurations and trim levels in a svelte design that is Strikingly Different™ and exciting to experience in person.
Prior to the start of LAIAS, the Mullen FIVE was selected as a finalist by the LA Auto Show for Top EV SUV in the ZEVA “People’s Choice” Awards.
LAIAS provides Mullen an opportunity to display multiple variants of the FIVE model while also showcasing its powertrain, battery and charging technology. The company intends to bring the FIVE to market in 2024, and reservations are currently open here.
Mullen’s development portfolio also includes EV Fleet Vans, which it intends to bring to market in Q2 2022, and the pure electric, high performance Mullen DragonFLY.
Expansion of Manufacturing Capacity
On November 2, 2021, Mullen announced plans to expand its facility in Robinsonville, Mississippi.
Mullen’s Advanced Manufacturing and Engineering Facility (AMEC) currently occupies 124,000 square feet of manufacturing space. The total available land on the property is over 100 acres, and Mullen is moving ahead with plans to build out another 1.2 million square feet of manufacturing space to support class 1 and class 2 EV cargo vans and the Mullen FIVE EV Crossover.
On the expanded site, Mullen plans to build a body shop, a fully automated paint shop and a general assembly shop.
EV Market Outlook
The global EV market was reported to consist of 3,269,671 units in 2019, a figure that is expected to grow at a CAGR of 21.1% through 2030 to a total of 26,951,318 units worldwide. This market’s monetary value was estimated at $162.34 billion in 2019 and is expected to grow at a CAGR of 22.6%, resulting in an approximate value of $802.81 billion by 2027. The primary driver for this exponential growth is a worldwide increase in vehicle emissions regulations.
Management Team
David Michery is the CEO and Founder of Mullen and has been leading the company and its divisions since inception in 2014. With over 25 years of executive management, marketing, distressed assets, and business restructuring experience, Mr. Michery brings a wealth of relevant knowledge and expertise to the Mullen brand. He has notably created 12 trademarks so far to develop the company brand and vision.
Mr. Michery is working toward a sustainable future accessible to all by creating a suite of clean-energy electric vehicles at varied price points. With entirely U.S.-based manufacturing and operations, he is also determined to have Mullen Technologies play a role in shaping a self-sustaining local economy by creating more jobs in America.
Mr. Michery manages risks and company expectations as a pathway to success and has personally overseen several businesses that totaled over $1 billion in transactions. His key strength is the ability to be fiscally responsible and lead teams to complete projects on time and within budget. As a seasoned professional in this space, Mr. Michery has demonstrated skill in building businesses from the ground up and into successful entities that subsequently sold for hundreds of millions of dollars.
Nextech AR Solutions Corp. (CSE: NTAR) (OTCQB: NEXCF) Nextech AR Solutions Corp. (CSE: NTAR) (OTCQB: NEXCF), based in Vancouver, Canada, is a leading provider of web-based augmented reality for e-commerce, advertising and virtual events, with technology ranging from simple 3D images to using 360-degree videos. Nextech AR provides businesses with a powerful end-to-end augmented reality platform designed specifically to increase online sales. The company is currently pursuing four unique verticals with its innovative technology, including: Unique Marketing Strategy Nextech AR’s efforts to disrupt the market for web-based augmented reality for e-commerce are supported by a unique go to market strategy. First, the company seeks to build or acquire platforms targeting a number of rapid growth industries, most notably AR, edTech, e-commerce, 3D/AR advertising and virtual & hybrid events. After identifying these market opportunities, the company seeks to integrate new AR technologies into existing or novel platforms in an effort to secure market share and promote growth. These technologies include WebAR, Human Holograms, 360 Portals, ScreenAR, Genie in the bottle and AiRShow. Nextech AR then aims to leverage these platforms to land and expand partnerships with a number of blue chip customers. The company’s current customer base includes the likes of Amazon, Johnson & Johnson, ViacomCBS, Toyota and Carnegie Mellon University. Growth Capital Nextech AR generates revenue through a software-as-a-service model from technology services, delivery of service revenue and sales of products through e-commerce. As noted in its latest investor presentation, the company achieved record bookings in Q4 2020 of $7.3 million (estimated), marking a greater than 275% year-over-year increase. The company also realized greater than 235% revenue growth for calendar 2020, reporting $20 million for the 12-month period. Nextech AR attributes its 2020 increase in revenues to the contracts secured with new customers, expanded agreements with existing customers and additional conversions from e-commerce channels. With its newly launched 3D ad network now bolstering its operations, Nextech AR is projecting revenues in excess of $50 million for 2021. Recent Company Highlights Management Team Evan Gappelberg is CEO and Founder of Nextech AR. He is an experienced operating executive specializing in creating, funding and running hyper-growth startups in both the public and private sectors. Notably, he took Take-Two Interactive Software Inc. (NASDAQ: TTWO) public with a market cap of $30 million and played a key role in guiding its growth to a current market cap of roughly $14 billion. Mr. Gappelberg has extensive experience as both a hands-on operating executive and a public markets professional. Paul Duffy is the company’s President. He is a serial entrepreneur with over 25 years of experience in successfully starting, expanding, diversifying and selling global technology companies. Mr. Duffy is the creator of the HumaGram and inventor of the patent for Holographic Telepresence over the Internet (TOIP). Augen Winschel is the COO of Nextech AR. He is an 18-year SAP executive with over 20 years of leadership experience in the areas of business management, business operations, marketing, product management, digital business and enterprise artificial intelligence. Kashif Malik, CPA, CA, is the company’s CFO. He has over 15 years of financial experience spanning IPOs, M&A activity, corporate restructuring and capital raising. Mr. Malik has worked globally with public and private companies, including Merck & Company Inc. (NYSE: MRK), Real Matters Inc. (TSX: REAL) and Constellation Software Inc. (TSX: CSU). He obtained his Chartered Accountant designation while working at Deloitte. Hareesh Acchi is the company’s President of 3D/AR Advertising. He is a 20-year Microsoft technology veteran with experience leading digital transformation and scaling businesses and enterprise organizations across the advertising industry.
Nowigence Inc. (OTCQB: NOWG)
Nowigence Inc. (OTCQB: NOWG) is an innovative software-as-a-service (“SaaS”) company focused on developing and bringing to market Pluaris™ — a comprehensive, ready-to-use artificial intelligence (“AI”) platform. Pluaris delivers the combined power of an intelligent reader along with a smart search engine. It works 24/7 reading and analyzing relevant content as is being created in various public and private data sources on topics that one reads either because one enjoys them or because one needs to gather information to fulfill job tasks or other responsibilities.
A personal knowledge management (“PKM”) tool, Pluaris is created for those who want to advance their competitiveness with the power of intelligent reading while searching for information. It accelerates the pace of problem-solving and decision-making. Pluaris is an end-to-end, fully automated, data-science product, offering data-at-scale capabilities mimicking the human process in their abilities to learn. It performs tasks that typically require human intensive activities when reading information from public and private data sources. It provides precise answers to questions asked, analyzes different perspectives, discovers new connections, and creates organized and nested notes. As a result, Pluaris allows teams to work collaboratively from anywhere in the world to share and draw informed conclusions.
Nowigence puts the power of data science into the hands of consumers through integrating state-of-the-art data processing techniques in an intuitive interface at an affordable subscription price. The Pluaris platform generates a trove of critical information to assist individuals, teams, and organizations to quickly build expertise. It reads and analyzes hundreds of pages in a few minutes wherein the data is transformed, linked, taxonomized, and optimized for storage and further trend analysis.
Key Target Market

Pluaris is pre-built with supervised and unsupervised machine learning (ML) and natural language processing (NLP) models. It has three main features. They are:
- A precise information retrieval engine
- An intelligent reader, and
- A Smart Search Engine that intelligently reads and analyzes content written inside files instead of contemporary search tools that use keywords to bring forth URLs or files which users still need to open to read manually.
Pluaris enables distillation of knowledge from hundreds or thousands of sources in seconds rather than the time-consuming method of reading and gaining insight from one source at a time. Keyword-based search-and-retrieval applications do not have the capability to open documents, read content, extract key points, conduct cause-and-effect analysis or answer questions specifically. Pluaris includes those features while going one step further with its semantic capabilities to empower users with interpretations of retrieved information. Nowigence estimates this feature alone can save typical researchers between one and three hours per workday. The platform also extracts only important and relevant information on every monitored or researched topic, reducing “noise” and information overload, a major source of workplace stress.
Pluaris Solution
The Problem
In the modern world, there is a need to consume a tremendous amount of text and transcript-based information for both personal and professional use. This need is met with exceptional challenges due to:
- Information Overload: For virtually any significant topic, the amount of textual information available and continually generated is vastly more than can be consumed by an individual.
- Pervasive Distractions: In the era of modern technology, new inputs such as instant messages, e-mails, social media and more reduce attention spans often lead to the disregard of information considered too long and complex to read.
- Highly Imperfect Human Recall: Information that is read is easily forgotten and, while key insights may be retained, the details are almost certainly lost.
- Data Sharing/Translation: Working in teams often finds colleagues researching the same content, with much of the information acquired by one individual lost in translation with the communication process to others.
Pluaris
From its early days, Nowigence has worked with pioneers and stalwarts in the fields of machine learning (“ML”) and natural language processing (“NLP”). In doing so, the company was keen to solve the big problem of the information age — too much data exists for it to be processed manually.
Pluaris is designed as a user-friendly platform, requiring no technical expertise or extensive training while avoiding structured or rigid methodology. As a result, Pluaris is adaptable to the unique needs by which individuals absorb knowledge and may be utilized across a variety of different functions and sectors. By leveraging the capabilities of Pluaris’ state-of-the-art no code editing, organizations have the flexibility to improve and tailor results without hiring data scientists. Further, real-time information retrieval, meaning instantaneous delivery of outputs at the click of a button, ensures the client never misses any piece of intelligence.
Pluaris does not simply gather information. The platform was built over the course of three years by a Nowigence team of experts and is designed to understand the context of every sentence it reads. In cases where Pluaris does make an error in contextual interpretation, the user can correct it, instantly giving the correction precedence over the ML’s algorithmic outputs. This removes the biggest criticism against AI/ML platforms, which is that annotating data and developing training datasets to build models takes too much time and effort from internal teams.
Use Cases
There is a virtually unlimited potential for Pluaris to cut through information overload and bring critical data to individuals and enterprises. Nowigence has customized its platform to cater to the needs of a variety of users, including an enterprise customer interested in tracking news and events in the telecom industry. In less than a week, Nowigence was able to quickly create and fine-tune a list of monitored topics, resulting in the team’s access to an annotated and industry specific news feed.
In another instance, Pluaris was useful to an employee of one of the world’s largest aluminum mining companies tasked with preparing talking points for her manager for an upcoming investor meeting. Using the platform, she built a comprehensive database of documents, including notes, transcripts, speeches, question-and-answer sessions, annual reports, and internal documents, some of which were from previous investor meetings. Through the platform’s dashboard and using various filters, she was able to quickly explore that database and pull the information together in a Pluaris Notebook to share directly with her supervisor.
Pluaris has further proven valuable in individual and personal-use cases. For example, a customer who was already using the platform for business intelligence decided to use his account to make improvements in his health after he received a report from his doctor of a high fasting blood sugar level. After uploading a few research reports, reviewing the summaries and exploring the annotated labels, the user set up Pluaris to monitor topics such as “lowering fasting blood sugar” and “low glycemic food.” From those results, he built an action list of daily habits for diet and fitness and, within two months, brought his fasting blood sugar level back down.
Market Considerations

Pluaris users include:
- Educators, Learners, and Publishers of Interactive Community Knowledge Banks: In a recent report titled “Digital 2022 – Global Overview,” published by the Tribune, it is said that the world between the ages of 16 and 64 spends a daily average of seven hours on the internet. Wikipedia estimates this age group to be 74% of the world’s population of nearly 8 billion people. Pluaris is helping create interactive community knowledge banks for people to save time and improve their quality of learning for specific subjects or topics. The first one being set up is on “healthy living.”
- Knowledge Workers: Gartner estimates there are more than one billion knowledge workers worldwide as of December 2019. According to a McKinsey report, employees spend 1.8 hours every day searching and gathering information. Nearly all data generated in businesses is in the form of language, but few today have the resources to leverage it. Pluaris, with its basis in data science, synthesizes knowledge from information assisting users to accelerate the pace of problem-solving and decision-making.
Nowigence offers subscription-based pricing to educators, researchers, learners, and publishers of community knowledge banks. Subscription prices are nominal but vary depending upon the volume, variety, velocity, and frequency of accessing large data sets.
Prices vary depending on the scope for small, medium, or large organizations. Typically, the business model is a one-time set up fee to taxonomize and link extracted entities which are user specific jargon. An additional fee is charged for scoping and integrating Pluaris with currently used enterprise tools. Subsequently, a monthly subscription fee is charged.
In 2020, the amount of data in the world was estimated at 44 zettabytes. By 2025, the amount of data generated each day is expected to reach 463 exabytes globally. Reading intelligently while searching for information is going to become an even bigger problem. Nowigence is targeting opportunity across the rapidly growing cognitive computing and personal knowledge management (“PKM”) markets. According to Allied Market Research, the cognitive computing market alone was valued at $8.87 billion in 2018 and is projected to reach a value of $87.39 billion by 2026. This represents a CAGR of 31.6% from 2019 through the end of the forecast period.
Leadership Team
Anoop Bhatia
Founder and Chief Executive Officer
Anoop Bhatia founded Nowigence Inc. and has served as its CEO since 2015. Previously, he worked as a global operation strategic transformation leader for Momentive Performance Material (formerly GE Silicones). Bhatia’s experience includes more than two decades serving in roles for various General Electric companies across different countries, including the U.S., India, The Netherlands and Germany. He played a key role in establishing GE Silicones as the first-ever wholly owned foreign subsidiary established in India in 1996. Bhatia holds a B.S. degree in chemical engineering from BITS in India and completed post-graduate studies in management from Heriot-Watt at Edinburgh in Scotland.
Gordon Haupt
Chief Technology Officer
Gordon Haupt brings more than 20 years of experience building and leading diverse engineering and operations teams, as well as a strong technical background in machine learning, signal processing and statistical data analysis. This includes expertise in speech and text, biotechnology and computer vision applications. He is experienced in all phases of engineering development and operations and is a named inventor on 15 issued patents. Haupt holds a B.S. degree in engineering mechanics from the University of Wisconsin and M.S. and Ph.D. degrees in aeronautics and astronautics from Stanford University.
David Evans
Chief Financial Officer and General Counsel
As an attorney and licensed CPA in the state of New York, David Evans has extensive experience in multistate and international tax policies and guidelines, federal taxation laws, mergers and acquisitions, including valuation of closely held businesses. He is a contributing author to the New York State Tax Service, a six-volume publication of NYS tax laws and regulations. His prior experience includes serving as a managing director for UHY Advisors LLC, a board member and chairperson of the Tax Division Executive Committee of New York State Society of Certified Public Accountants, as well as a past president of the Estate Planning Council of Eastern New York. Evans holds degrees from Hofstra University and State University of New York at Buffalo.
Uday Bawa
Vice President Business Development – India, SEA, and MEA
Uday Bawa brings a wealth of nearly two decades of global P&L experience, growing businesses from scratch in the field of energy and sports. He has worked in B2C and B2B sales environment focusing on product sales, building the brand and generating endorsement deals for renowned sports personalities in India. Previously, he worked in the petrochemicals industry, where he led Houston-based Dorf Ketal’s Fuels business for North America. He was instrumental in generating sponsorship revenues for the Commonwealth Games in Delhi, India, in 2010 from corporate sponsors. He has also managed Indian sports icon Sachin Tendulkar’s commercial interests and endorsement deals. He completed his bachelor’s from Symbiosis International University in India and then his executive MBA from Rice University at Houston in Texas. He is a Doon school (India) alumnus.
Odyssey Group International Inc. (OTC: ODYY)
Odyssey Group International Inc. (OTC: ODYY) is a medical technology company focused on developing lifesaving medical products that offer technological and clinical advantages over current standards of care.
The company’s portfolio of product technologies is diverse, featuring four unique medical products in development. Odyssey’s goal is to deliver superior products with enhanced clinical utility and market potential, thereby yielding a high rate of return for its shareholders and partners. It is guided by a senior management team with significant experience relating to refining technologies, building commercial systems and forging strategic partnerships.
Product Portfolio
Pharmaceuticals
Odyssey has two pharmaceutical products in development:
- PRV-002 is a novel compound for the treatment of concussion, which currently has no FDA-approved drug. In pre-clinical studies, PRV-002 has been shown to significantly improve both neuroscore and memory score following injury in rats subjected to concussion models. Importantly, the first-in-class novel neurosteroid demonstrated no drug-related toxicity in these trials.
- PRV-002 is currently being evaluated in a phase I clinical trial for the treatment of concussion, with phase II trials planned for launch in Fall 2022. Odyssey has also highlighted the potential of PRV-002 for additional indications such as Alzheimer’s disease, Parkinson’s disease, ALS and chromic traumatic encephalopathy (CTE).
- PRV-001 is a novel compound intended to treat Niemann-Pick disease, a rare neurodegenerative-lysosomal storage disorder that affects an estimated 1 in 150,000 individuals in the U.S., demonstrating a 5x higher incidence in Middle Eastern populations.
- Odyssey expects to receive Orphan Drug designation from the FDA for PRV-001, which would accelerate its pathway to FDA approval and provide seven years of market exclusivity.
Medical Devices
Odyssey is also developing two medical device candidates:
- CardioMap® is intended to provide early, non-invasive testing for heart disease. The system offers a number of potential advantages over traditional EKGs, including requiring less training to operate, offering heightened sensitivity and coming in a small and portable form factor. CardioMap is being developed for a 510(k) regulatory pathway, which requires a study to demonstrate equivalence to legacy EKG offerings.
- When approved, CardioMap is expected to be the only device in its class that has a predictive value, illustrating ‘grey’ areas where deterioration has begun but not yet led to pathology. Odyssey expects this feature to provide a powerful incentive for doctors to use the CardioMap device in end markets such as hospitals, doctors’ offices, rehabilitation centers and sports medicine practices.
- Save-A-Life (SAL) is a patented, single-action, instantaneous, handheld, mechanical anti-choking device that creates a vacuum chamber in the mouth to dislodge throat obstructions in a matter of seconds, all without harm to the victim. The device is currently in development, with a proof of concept established.
- Odyssey believes that, once FDA-approved, its anti-choking device will quickly become the “accepted” standard and leader in the treatment of choking incidents globally. Its low-cost manufacturing and convenient portable design give SAL a competitive edge over competing devices utilizing cumbersome masks.
Market Opportunities
Odyssey’s varied development pipeline positions it to address a number of sizable market opportunities with significant unmet medical need. Concussions alone currently account for medical costs of roughly $10-15 billion annually in the U.S., despite the lack of a currently approved FDA drug treatment. This need is particularly apparent in the military and sports industry, where the likelihood of athlete head-injury recurrence is estimated at 75%.
It is for this reason that, in March 2021, Odyssey announced the formation of a sports advisory board featuring well-known athletes supporting the company’s efforts to enhance public awareness of traumatic brain injuries and concussions, as well as the need for an FDA-approved therapy. Members of Odyssey’s sports advisory board include NFL Hall of Famers Kurt Warner & Brett Favre and two-time Olympic gold medalist Abby Wambach.
With its CardioMap platform, Odyssey is targeting the global cardiac monitoring market, which was valued at $28 billion in 2021 by Insight Partners and forecast to reach $43 billion by 2028.
Save-A-Life targets a similarly underserved market. Choking is the fourth-leading cause of death in children, and approximately 5,000 choking deaths occur each year in the U.S. While 95% of these deaths result from in-home incidents, current choking rescue devices fail to address in-home applications.
Management Team
Joseph Michael Redmond is the President, CEO and Chairman of Odyssey. He has over 30 years of commercial experience in medical device companies, previously serving as CEO of Parallax Health Sciences Inc., V.P. of Business Development for DxTech Inc. and V.P. of Sales and Marketing for Bioject Medical Technologies Inc. While at Bioject, Mr. Redmond helped raise over $15 million in capital, entered into several licensing and distribution deals with major biotech and pharmaceutical companies and grew the market cap of the company from under $10 million to over $400 million. He started his career at Abbott Labs and holds a B.A. from Denison University.
Christine M. Farrell is the company’s CFO and Secretary. Prior to joining Odyssey, Ms. Farrell was Vice President of Finance for Bioject Medical Technologies Inc. She also held accounting and financial management positions with Spar-Tek Industries, a manufacturer of high quality and cutting-edge technology for the plywood industry, and Action Machinery, a seller of new and used robotic machine tools and equipment. Ms. Farrell holds a B.A. in Accounting from the University of Washington and an M.B.A. from Willamette University.
Dr. Jacob W. Vanlandingham is Odyssey’s Head of Drug Development. Dr. Vanlandingham holds a Ph.D. in neuroscience with a molecular biology focus. He is a member of the Society for Neuroscience, American Society for Nutritional Sciences, National Neurotrauma Society, Faculty for Undergraduate Research in Neuroscience and the International Association of Medical Science Educators.
Perpetual Industries Inc. (OTC: PRPI)
Perpetual Industries Inc. (OTC: PRPI) is an incubator for the development of innovative, energy-efficient technologies aimed at commercializing products that have the potential to impact and advance a wide range of industries on a global scale.
The company’s team of experts and trusted industry partners have the resources to provide essential components needed to take projects from their initial stages through to the end products. Perpetual Industries values strict controls and high levels of quality through all stages of research, development, manufacturing and commercialization.
Perpetual Industries was founded by President, Chairman and CEO Brent W. Bedford in 2005. It is located in Auburn, Indiana.
R&D Portfolio Overview
Perpetual Industries is expanding expertise and knowledge of energy-efficient technology by developing low-cost, green energy solutions for various industries, including artificial intelligence, blockchain mining, graphic rendering, renewable energy, cloud computing and internet of things (IoT), all while continuing research, development and commercialization of its proprietary XYO Balancing Technology in key applications.
XYO Balancing Technology
XYO Balancing Technology delivers high-performance solutions for inefficiencies that commonly affect rotating equipment, machinery and devices. It is designed to harness rotor displacement energy to move compensating masses and automatically correct for imbalances, effectively reducing vibration.
Key highlights of the company’s XYO Balancing Technology include:
- Customized XYO balancers can be created for almost everything that rotates, providing virtually unlimited potential applications.
- XYO Balancing Technology is optimized specifically to eliminate vibration in rotating equipment and enable environmentally responsible products to operate more efficiently.
- Leveraging a proprietary design, XYO Balancing Technology is the result of over 25 years of research and development effort.
WindSilo – Vertical Axis Wind Turbine
Implementing its proprietary XYO Balancing Technology, Perpetual Industries’ WindSilo turbine improves balancing issues that are common in most wind turbines today. The company’s design is engineered to allow for much faster spin speeds and greater energy output.
The company believes that this innovative turbine design could eliminate the expensive traditional methods of balancing wind turbines while increasing their performance, reliability and efficiency.
In November 2020, Perpetual Industries announced that Trine University, a private post-secondary institution located in Angola, Indiana, had been awarded a grant to assist the company in the development of the WindSilo.
The XYO Washing Machine
Perpetual Industries is currently developing a proprietary domestic washing machine design implementing XYO Mechanical Balancers to dynamically compensate for variable mass imbalance during the spin cycle. The company expects these efforts to produce a number of benefits, including higher spin speeds, reduced energy consumption, decreased noise emissions and less mechanical wear & tear.
The company’s research shows tremendous market potential for a more efficient washing machine design. With an estimated 70 million washing machines produced annually and over 500 million used daily, even a small reduction in energy consumption could be pivotal. Reducing energy usage of all washing machines by just 15% would save enough energy to power the city of Milan. Perpetual Industries’ energy efficient design is expected to reduce energy usage by up to 50%.
Prototype testing of Perpetual Industries’ design has established the XYO Washing Machine as highly effective at reducing vibration when built into the spin basket assembly.
Green Energy Mining System
Using its expertise and knowledge of environmentally friendly technologies, Perpetual Industries is developing low cost, environmentally responsible energy solutions for powering large scale blockchain mining operations.
The company’s Green Energy Mining (GEM) System is being hailed as the next generation of energy efficient cryptocurrency mining. Powered by renewable & surplus energy sources such as wind, solar, natural gas, wind and geothermal that utilize battery storage technology, the platform addresses rising demand for computing power.
Renewable Energy Market Outlook
The global renewable energy market was valued at $928 billion in 2017 and is expected to continue expanding at a CAGR of 6.1%, resulting in a value of $1.5 trillion by 2025, according to Allied Market Research (https://ibn.fm/C06xF). Hydroelectric power is projected to be the most lucrative segment of the entire global renewable energy industry, followed by the wind, bioenergy, solar and geothermal segments.
Worldwide Auctioneers Acquisition
In January 2021, Perpetual Industries announced its acquisition of The Worldwide Group LLC, operating as Worldwide Auctioneers.
Worldwide Auctioneers is a U.S.-based boutique auction firm specializing in the sale and acquisition of classic vintage motorcars at auction around the globe. With an impressive 20-year history and a talented team, Worldwide offers an extensive range of personalized services to collectors, including private sales, appraisal, collection direction and consultancy, estate planning and asset management.
Perpetual Industries expects Worldwide to benefit from multiple channels of collaboration moving forward, particularly within the company’s blockchain division.
Classic Car Market Outlook
The U.S. classic car market has recorded steady expansion over recent years, accounting for revenue of approximately $12.63 billion in 2020, according to Statista (https://ibn.fm/Fydhj). The same report forecasts growth to $15.52 billion by 2023, representing a CAGR in excess of 7 percent. Classic car dealers in the U.S. have cornered a significant portion of this market opportunity. According to data from IBISWorld (https://ibn.fm/k30F5), the market for classic car dealers in the U.S. was valued at $2.1 billion in 2021, achieving a CAGR of 1.5% from 2016 to 2021, despite the challenges associated with the COVID-19 pandemic.
Management Team
Brent W. Bedford is the President, Chairman, CEO and founder of Perpetual Industries. He has held these roles continuously since founding the company in January 2005. He has a deep understanding of every aspect of the company’s business, products and markets. He also has experience developing corporate strategies, assessing emerging industry trends and carrying out business operations. Mr. Bedford has a strong background in mechanical applications, with expertise in finance, private startups, public startups and corporate turnarounds.
Carl Dilley is Director and COO of the company. He is a career entrepreneur who has served as a C-level officer in many different companies across multiple industries. Mr. Dilley has been instrumental in taking over 400 companies public. He has been involved in the investment industry since 1983. He has held FINRA series 24, 66 and 7 Securities licenses, allowing him to perform retail, investment, banking and new listing services functions.
William Griffin Thomas, CPA, is the company’s CFO. He holds a Bachelor of Science in Accounting from the University of Tampa and a Bachelor of Science in Agribusiness from the University of Florida. Mr. Thomas is a licensed CPA with over 19 years of experience spanning both the private and public sectors, as well as time with non-profits. His expertise includes auditing, budget analysis, fixed assets, financial modeling, SEC financial reporting, GAAP compliance and fair value measurements.
PlantX Life Inc. (CSE: VEGA) (Frankfurt: WNT1) (OTCQB: PLTXF)
PlantX Life Inc. (CSE: VEGA) (Frankfurt: WNT1) (OTCQB: PLTXF) aims to redefine the plant-based community through e-commerce, with a core objective of becoming the most trusted and convenient destination for people living plant-based lives. PlantX is a multifaceted marketplace providing consumers all things plant-based ranging from an efficient e-commerce experience, connecting consumers with interactive PlantX brick-and-mortar stores, and a PlantX home delivery system for products, meals, recipes and more.
PlantX is a high-growth technology company focusing on consumer-packaged goods (“CPG”) for the plant-based opportunity. The PlantX platform aims to serve as the digital face of this community with its one-stop-shop for everything plant-based, including:
- An easy-to-use e-commerce shopping experience featuring the following:
- Plant-based grocery items (from all your pantry needs to vitamins, cosmetics and even pet food)
- Meal delivery with recipes created by well-known plant-based chefs throughout the world
- Plant shop – delivering a wide variety of affordable indoor houseplants to homes across Canada and the U.S.
- Easy to follow plant-based recipes every week
- Partnerships with restaurants, nutritionists, chefs and brands
- A community of like-minded individuals
- State-of-the-art flagship PlantX locations
Since first launching in February 2020, PlantX Life has offered various services available through its comprehensive platform. This online marketplace features over 10,000 items across diverse product categories such as pantry items, beverages, personal care, pet food and indoor plants. In addition, PlantX has collaborated with renowned chefs and nutritionists to create 20 unique and pre-made meals delivered to the comfort of your own home.
Headquartered in Vancouver, Canada, PlantX’s mission is to spearhead the plant-based movement, celebrate and promote health and wellbeing, raise plant-based awareness in a hyper-palatable world, connect with global consumers and forge a welcoming plant-based community.
The company currently reports 4 million stock options and 24 million warrants outstanding, with a total of 88,832,159 shares issued and outstanding and a total market cap of $89.9 million on January 18, 2021. PlantX has continued to catalyze its capital markets dynamics by applying to list its common shares on the Nasdaq Capital Market (“NASDAQ”).
Market Outlook
With its comprehensive e-commerce platform, PlantX is strongly positioned for a prominent role in the fast-growing plant-based food market, e-commerce and the online food delivery sectors. The global plant-based food market is expected to reach $74.2 billion by 2027, expanding at a CAGR of 11.9%. Similarly, the online food delivery market has steadily grown, especially during the current pandemic. This trend seems here to stay. In the United States alone, the sector is expected to report $28.5 billion by 2024, with companies such as UberEats experiencing 152% increases in food deliveries in the summer of 2020.
Complementary to these trends, and as a result of the COVID-19 pandemic, online sales and digitization have also both grown exponentially in 2020. Grocery shopping has seen a remarkable transition to e-commerce, with online grocery sales growing by 53% in 2020. Amid the pandemic-imposed physical interactions and related consumer behavior change, large retailers have been compelled to meet this surge in e-commerce demand. For example, Whole Foods Markets has increased its online sales capacity by over 60% in 2020. The global meal kit delivery system is also becoming increasingly popular and is expected to achieve a market value of $19.92 billion by 2027, expanding at a CAGR of 12.8%.
PlantX aims to capitalize on this anticipated exponential market growth of the plant-based, e-commerce and home-delivery industries.
Digital Platform for the Plant-Based Community
The digital interface provided by PlantX spans a health and wellness initiative that offers thousands of plant-based products, meal delivery, indoor plants, recipes and a community space for those who are like-minded about plant-based products and healthy lifestyles. PlantX has been compared to Amazon, except with a focused tailored selection of plant-based offerings.
PlantX provides everything a consumer needs for plant-based living at the click of a button. With PlantX, customers can:
- Shop
- Find recipes
- Read blogs
- Join a community forum
- Listen to podcasts
- View cosmetics
- Research vitamins
- Purchase plant-based pet foods
- Read corporate updates
- Subscribe to an insightful newsletter
The company’s website was designed with a user-friendly interface that allows customers to visit the site and easily find what they need. Forums for communicating with a plant-based community make it easier to swap recipes or locate the best restaurants serving vegan and vegetarian-friendly cuisine.
PlantX Flagship Locations – British Columbia (Canada), San Diego (California), & the State of Israel
PlantX will link the e-commerce platform to flagship brick-and-mortar stores for a highly sensory customer experience. This is anticipated to drive corporate growth and global brand recognition.
These PlantX branded flagship locations will first launch in:
Customer engagement, education and creating a global plant-based community will be furthered through this initiative.
PlantX Restaurant Partnerships
With consumers becoming better informed and more health and environmentally conscious, a growing number of restaurants will start catering to the needs of customers who are vegan, vegetarian, have food-allergies (or specialized diets), or simply want to eat healthier.
PlantX proactively aims to support this change and help restaurants meet the needs of the plant-based community. Restaurants that want to increase revenue, drive traffic and make an impact can therefore partner with PlantX to better serve their customers by expanding and refining their menus.
Future Goals for PlantX Life
Having successfully completed all of the milestones that PlantX had set-out to achieve in the second half of 2020, PlantX strives to continue scaling through organic growth, strategic partnerships and accretive M&A opportunities. The upcoming plans from PlantX includes a global expansion strategy for distribution in North America, Europe and Israel.
Verticals launched in 2020 include:
- New meals and programs by renowned chefs
- Flagship PlantX locations
- PlantX branded goods
- United States meal delivery and LIV
- Online peer-to-peer fitness
Management Team
Sean Dollinger, the Founder of PlantX Life Inc., has had a very active professional career that started when he was only 17. While still in college, he started a delivery service that soon became one of Canada’s largest delivery firms (before companies like Postmates and Uber Eats ever existed). In 2014, Mr. Dollinger founded Namaste Technologies, the largest international e-commerce distributor of vaporizers and accessories. He brought Namaste public and turned it into a $1.2 billion business in two years. After finding a plant-based diet himself, and seeing the massive benefits that it provided for him, he decided he wanted to find a way to give back to the community and focus on something he loves. PlantX Life was born from this desire and became his passion project. He truly walks the talk.
Julia Frank is the CEO of PlantX Life. She has an MBA in digital entrepreneurship, and, in her past roles, she set up renowned strategies for large corporations like BMW and Daimler in Germany. Beyond her professional business prowess, Ms. Frank finds tremendous joy in preparing delicious and nutritious plant-based meals and is the face of the company. She practices a healthy and active lifestyle that includes experiencing as many cultures as possible to add more knowledge of the industry at large. This globally inclusive perspective gives her the unique advantage of being able to see plant-based living from all angles.
Lorne Rapkin, CPA, CA, LPA, is the President and CFO of PlantX Life and is also a partner at Rapkin Wein LLP. He has experience with clients in almost every industry, including finance, professional services, real estate, automotive, media and manufacturing. Mr. Rapkin works very closely with investment and public firms, seeking to comply with IFRS accounting standards. His roles often require him to work with management on go-public transactions, acquisitions and mergers. His keen attention to detail is an asset to any client he works with, and PlantX is no exception.
Alex Hoffman is the company’s CMO and has spent the last 10 years in the creative field cultivating her passion for design and appreciation for beauty. This is apparent in all of the creative decisions and outcomes seen at PlantX. Her role within the company is to oversee all of the brand marketing activities, establish and execute key processes for rapid growth, and work closely with management to refine the brand’s message for key segments and emerging opportunities. She has a sharp vision for exactly what’s needed to convey the company’s core messages and principles to both the public and investors, and she is a visionary with respect to creative marketing ideas and concepts.
Playgon Games Inc. (TSX.V: DEAL) (OTCQB: PLGNF)
Playgon Games Inc. (TSX.V: DEAL) (OTCQB: PLGNF) is a SaaS technology company focused on developing and licensing digital content for the growing global iGaming market. The company provides a multi-tenant gateway that allows online operators the ability to offer their customers innovative iGaming software solutions. Its current software platform includes Live Dealer Casino, E-Table Games and Daily Fantasy Sports. Seamless integration at the operator level allows customer access without requiring the sharing of any sensitive customer data. Playgon games run on any browser and any device as fast and secure as a native app, without requiring any app store download. All that’s needed is a stable internet connection. The gaming experience is identical across all mobile devices. As a true business-to-business digital content provider, the company’s products are scalable turnkey solutions for online casinos, sportsbook operators, location-based operators, media groups, and big database companies.

Playgon’s proprietary technology provides digital games for online gambling sites and mobile device apps, with the company licensing its mobile live-dealer technology to online gaming operators worldwide. Playgon combines high definition live streaming dealers with state-of-the-art augmented reality betting to provide the most authentic casino experience, live from Las Vegas. Playgon’s mobile platform features popular table games, all optimized for one-handed play on mobile devices.
The COVID-19 pandemic has accelerated an already existing shift away from location-based casinos to online gambling. At the same time, the proliferation of mobile devices has provided players with new access to betting. A younger, tech-savvy consumer demographic is driving adoption of digital gaming globally. To meet this demand, Playgon has launched a studio with 10 gaming tables from which its live dealer streaming video originates. The company’s platform is live with multiple online casino operators through four aggregator clients in South Africa and Europe, and commitments are coming in from more.
Playgon plans to expand the studio to 25 tables in the near term and is working to establish a U.S. strategy. The company will continue to expand licensing of its live dealer games to iGaming operators worldwide under a SaaS license agreement. As a B2B software supplier, Playgon avoids player acquisition costs.
Games

Live Dealer Casino
Playgon offers the first and only Live Dealer Casino streaming live from Las Vegas. The company brings cutting-edge handheld features and functionality to the mobile generation of gaming enthusiasts who demand a world-class gaming experience on all devices. Playgon’s Blackjack delivers the look and feel of location-based casino tables with features providing players with the most unique user experience. The company’s true-to-life Roulette offers players a clear and uninterrupted view of the dealer, wheel, ball, bets, results, trends and statistics. Players can strategize, place multiple bets, track results and review trends without ever losing focus of the game.
Playgon’s traditional Baccarat and proprietary Tiger Bonus Baccarat™ prove their worth by not only recognizing the need for a prominent product, but by adding elements which separate them from the pack without removing their authenticity. The games mix advances in technology with the traditional game attributes that have resonated and captivated players for hundreds of years.

eTable Games
To lead the rise of mobile-first gaming, Playgon developed a user experience perfected for one-handed play. Providing this next evolution in gaming technology ensures the company’s client operators loyalty from existing customers and is a powerful strategy to attract and retain new players. Playgon’s VEGAS LOUNGE™ brings together an innovative mix of games, technology and gameplay that offers players an authentic experience and real Las Vegas casino fun every time, everywhere.
Daily Fantasy Sports
Playgon’s Daily Fantasy Sports (DFS) are a subset of fantasy sport games which typically target a younger demographic. DFS provides iGaming operators a turnkey fantasy sports platform that can quickly go to market, integrate with the operator’s existing operations and services, and be customized to match and enhance the operator’s brand. The platform is mobile and desktop friendly, built for regulated market environments, and allows operators to monetize users through a network of shared liquidity.
Market Outlook
Online casinos and sports betting sites/apps are increasingly adding market share to traditional location-based casinos. This trend is only expected to accelerate as millennials reach their peak earning years and Gen Z youth begin to complete their education and move into careers. These generations are completely comfortable with online recreation, as well as tech like digital wallets and digital gameplay that underpins Playgon Games. The company has been described as “Netflix + Vegas, all in one.”
The online gambling market is slated to reach a value of $127.3 billion by 2027, according to Grand View Research, with much of the growth expected from the U.S. and Asia. Even Europe, the most mature gaming market, is expected to grow at a rate of 20-25 percent year over year. The current global online Live Casino TAM is estimated at about $6 billion annually, and revenue is forecast to reach more than $8 billion by 2023 and more than $13 billion by 2027.
Management Team
Darcy Krogh is CEO of Playgon Games. He is a veteran of the iGaming industry with over 20 years of experience. In 1999, he co-founded Chartwell Technology Inc., which pioneered the development of browser-based digital content for the iGaming industry. After that company was sold to Amaya Gaming Group, he served as VP Business Development with Amaya. In 2016, he started Playgon Games (formally Global Daily Fantasy Sports Inc.) as President and CEO. His experience in the online gaming industry includes sales and marketing, relationship management, corporate finance, M&A, and strategic corporate development.
Guido Ganschow is President of Playgon Interactive. He has more than 12 years of experience in creating real-time Live Dealer technology and platforms and was the co-founder and Creative Director for a Macau-based casino consortium. Between 2008 and 2014, he successfully created and established Live Dealer platform businesses in Asia and Europe, and executed commercial partnerships, sales, and integration of the Live Dealer solution with major global gaming brands, including Ho Gaming Group, Chartwell Technology and Amaya Gaming Group.
Steve Baker is COO of Playgon. He is a former VP Operations for Shaw Communications, where he was directly involved in video streaming, home entertainment, new products, sales and M&A. He oversaw revenue growth from $300 million to $2.8 billion and employee growth from 350 to 13,000. He has broad experience and a proven record in development and implementation of cost effective and efficient growth strategies transitioning businesses from development to operations.
Harry Nijjar is CFO of Playgon Games. He is currently a Managing Director with Malaspina Consultants Inc. and provides CFO and strategic financial advisory services to his clients across many industries. This experience has allowed him to help his clients successfully navigate the regulatory and financial environments within which they operate. Mr. Nijjar holds a CPA-CMA designation from the Chartered Professional Accountants of British Columbia.
PowerTap Hydrogen Capital Corp. (NEO: MOVE) (FWB: 2K6A) (OTC: MOTNF)
PowerTap Hydrogen Capital Corp. (NEO: MOVE) (FWB: 2K6A) (OTC: MOTNF) is an investment holding company that focuses on investing in and providing early-stage financing to both public and private businesses. Since its original listing with the Canadian Stock Exchange (“CSE”) on January 23, 2019, the company has made investments in a number of different businesses in a variety of industries, including the energy and cannabis sectors. As per the company’s investment policy, its primary goal is to identify and capitalize on high-return investment opportunities presenting the ability to achieve capital appreciation and liquidity.
PowerTap Hydrogen Capital continues to be opportunistic in evaluating prospects across the renewable energy, bio-medical, pharmaceutical and naturopathic sectors, both as an investor and as an operator. The company’s main focus at the moment is to identify such opportunities in the renewable energy industry, including wind, solar and geothermal power and hydrogen and fuel cell technologies, as well as in the biomedical, pharmaceutical and naturopathic sectors, which may include medical or recreational cannabis.
PowerTap Hydrogen Capital currently has 10 investments in a variety of sectors and successfully held nearly C$120 million in investments during the past fiscal year (https://ibn.fm/8oktZ). It returned capital to its shareholders through the distribution of its interest in AgraFlora Organics International Inc. in May 2020 (https://ibn.fm/FRAvq).
Headquartered in Vancouver, British Columbia, PowerTap Hydrogen Capital was formerly named Organic Flower Investments Group Inc. As of November 10, 2020, the company officially changed its name to PowerTap Hydrogen Capital and started trading on the CSE under new ticker symbol ‘MOVE’.
PowerTap Acquisition, Hydrogen Fueling Infrastructure Collaboration
In alignment with its updated investment policy, a reconstituted investment committee and a revised strategy to reflect its focus on the renewable energy market, PowerTap Hydrogen Capital recently completed the acquisition of a 90 percent equity interest in California-based PowerTap Hydrogen Fueling Corp.
Leveraging an impressive portfolio of IP and advanced deployed technologies developed over two decades via substantial investments and partnerships, PowerTap is working on building and expanding a hydrogen filling station network, initially across North America. The company believes that its platform has a significant advantage over other hydrogen fueling stations, because it has a smaller physical footprint and further has the capacity to produce hydrogen fuel on site. As most other hydrogen fueling stations buy hydrogen for storage at higher costs, PowerTap’s model is believed to be exponentially more cost-effective and expandable.
PowerTap Hydrogen Capital’s investment and acquisition will allow PowerTap to step up its efforts and begin work on the hydrogen fueling station network in stages, starting with engineering and design, ongoing development of PowerTap’s third generation product and, finally, licensing & permitting and site preparation. Development is expected to begin in Q4 2021 with engineering and design. Overall, the initial portion of the project is expected to cost $17 million, with PowerTap Hydrogen Capital and PowerTap planning to secure government financing and credit, as well as equity, debt and convertible debt offerings, to fund the infrastructure’s development.
PowerTap technology is already deployed across multiple hydrogen fueling stations in public and private enterprises spanning California, Maryland, Massachusetts and Texas. The company plans to deploy its hydrogen fueling infrastructure at existing truck stops and gas stations across the country, beginning with up to 1,000 stations within the next three to five years. At the moment, there are roughly 70 active hydrogen fueling stations operational and available to consumers in the United States.
Hydrogen Industry Outlook
The project is expected to bring significant opportunities for PowerTap and PowerTap Hydrogen Capital on the fast-growing hydrogen market, driven by a worldwide focus on clean energies and environmentally friendly fueling solutions for the transportation industry.
Hydrogen-powered vehicles come with tremendous advantages over gas, diesel and even electric vehicles in terms of cost per mile, fueling time and driving range, as well as boasting significantly lower emissions. Well-established vehicle manufacturers such as Hyundai, Toyota, Daimler and Volvo are already including hydrogen-powered cars in their product lineups, and Nikola Motors has announced plans to manufacture hydrogen electric long-haul vehicles.
“As an experienced developer of technology in an important area that is finally having its time as a green but also economically compelling energy option, PowerTap is intent on becoming a leading part of the multi-billion dollar hydrogen fueling space,” PowerTap CEO Raghu Kilambi explained in a news release on October 28, 2020 (https://ibn.fm/oaXem).
A recent industry report developed by a coalition of major oil and gas, power, automotive, fuel cell and hydrogen companies indicates that the sector is expected to grow to $140 billion a year in revenue by 2030, creating 700,000 jobs in the U.S. alone (https://ibn.fm/UMI5q). According to Fuel Cell and Hydrogen Energy Association President Morry Markowitz, the sector could expand to $750 billion a year in revenue and 3.4 million jobs by 2050.
The U.S. is already engaged in the hydrogen economy, having more than half of the global number of fuel cell vehicles and investing hundreds of millions of dollars a year, but the country can greatly expand its global energy leadership by scaling up operations in the hydrogen economy, per the industry report.
With the upcoming change in administration in January 2021, the U.S. is expected to renew its commitment to clean energy. Moreover, the U.S. federal government is expected to invest significantly in clean energy and related infrastructure, including hydrogen, according to PowerTap.
“As the U.S. federal government has previously invested in the PowerTap technology, we are optimistic that we will have a seat at the table when USA clean energy/hydrogen infrastructure spending initiatives are designed,” Kilambi added.
Management Team
Joel Dumaresq is the CEO and interim CFO of PowerTap Hydrogen Capital. He is a proven executive with extensive operational and senior management experience in mining, energy and alternative energy, as well as the cannabis and hemp space. Dumaresq began his career in the corporate finance space, having spent 12 years with RBC Dominion Securities. He brings 30 years of experience in the financial sector to the company, has been instrumental in raising over $250 million in venture capital finance, and he has personally managed a number of successful public listings.
Brendan Purdy serves as a director of PowerTap Hydrogen Capital. An experienced businessperson who has led five different companies, Purdy brings years of experience in different industries, including cannabis, blockchain and data security, gaming, mining and energy, and finance and law. He received a graduate degree from the University of Ottawa and an undergraduate degree from the University of Western Ontario.
Theo van der Linde serves as a director of PowerTap Hydrogen Capital. He is a Chartered Accountant with over 20 years extensive experience in finance, reporting, regulatory requirements, public company administration, equity markets and financing of publicly traded companies. He has served as a CFO & Director for a number of TSX Venture Exchange- and Canadian Securities Exchange-listed companies over the past several years. His industry experience spans the financial services, manufacturing, oil & gas, mining and retail industries. More recently, van der Linde has been involved with future use trends of natural resources, as well as other disruptive technologies.
Raghu Kilambi is the CEO and CFO of PowerTap Hydrogen. He is a seasoned investor and entrepreneur with over 25 years of global business experience in public and private investments, building businesses and creating shareholder value. He has raised over $1 billion of equity and debt capital for private and public companies and been involved in many M&A acquisitions and exits.
Predictive Oncology (NASDAQ: POAI)
Predictive Oncology (NASDAQ: POAI) is a knowledge-driven precision medicine company focused on applying data and artificial intelligence (AI) to personalized medicine and drug discovery. The company applies its smart tumor profiling and AI platform to extensive genomic and biomarker patient data sets to build predictive models of tumor drug response to improve clinical outcomes for the cancer patients of today and tomorrow. The company has several tools that support its mission of bringing precision medicine to the treatment of cancer.
Through its subsidiaries, Predictive Oncology’s portfolio of assets includes the following:
- A database of clinically validated historical and outcome data from patient tumors
- An in-house Clinical Laboratory Improvement Amendments (CLIA)-certified lab
- A “smart” patient-derived tumor profiling platform
- An in-house bioinformatics artificial intelligence (AI) platform
- A new computerized approach growing tumors in the lab to rapidly develop patient specific treatment options
- An FDA-approved fluid collection and disposal system
Using these resources, and in collaboration with key players in the pharmaceutical, diagnostic and biotech industries Predictive Oncology is working to determine the best pathways for more individualized and effective cancer treatment.
Subsidiaries
Predictive Oncology leverages the synergies of its three wholly owned subsidiaries to bring precision medicine to the diagnosis of cancer.
Helomics applies artificial intelligence to its rich data gathered from the company’s trove of more than 150,000 tumors to personalize cancer therapies for patients as well as drive the development of new targeted therapies in collaborations with pharmaceutical companies. This database, the largest of its kind in the world, is comprised of ovarian, head and neck, colon and pancreas tumors. Helomic’s CLIA-certified lab provides clinical testing that assists oncologists in individualizing patient treatment decisions, by providing an evidence-based roadmap for therapy.
In addition to its proprietary precision oncology platform, Helomics offers boutique CRO services that leverage its TruTumor™ patient-derived tumor models coupled to a wide range of multi-omics assays (genomics, proteomics and biochemical), and an AI-powered proprietary platform (D-CHIP) to provide a tailored solution to its clients’ specific needs.
TumorGenesis is developing a new, rapid approach to growing tumors in the laboratory without the use of rats or mice, allowing for the identification of biomarkers indicative of cancer. This methodology “fools” the tumor into thinking it is still in the body. As a result, the tumor reacts as it naturally would, thereby increasing the accuracy of the biomarker. Once the biomarkers are identified, they can be used in TumorGenesis’ Oncology Capture Technology Platforms which isolate and helps categorize an individual patient’s heterogeneous tumor samples to enable development of patient-specific treatment options.
Skyline Medical’s patented, FDA-cleared STREAMWAY® System is the first true, direct-to-drain fluid disposal system designed specifically for medical applications such as radiology, endoscopy, urology and cystoscopy procedures. The STREAMWAY system is changing the way healthcare facilities collect and dispose of potentially infectious waste fluid by connecting directly to a facility’s plumbing system to automate the collection, measurement and disposal of waste fluids.
The STREAMWAY minimizes human intervention for better safety and improves compliance with Occupational Safety and Health Administration (OSHA) and other regulatory agency safety guidelines. The STREAMWAY eliminates canisters, carts and evacuated bottles, which reduces overhead costs and minimizes environmental impact by helping to eliminate the approximately 50 million potentially disease-infected canisters that go into landfills annually in the United Sates.
Skyline has achieved sales in five of the seven continents through both direct sales and distributor partners.
Competitive Advantage
Precision medicine has become the holy grail of cancer therapeutics. Data driven predictive models of tumors and their responses are critical in both new drug development and individualized patient treatment. The race has begun to model various tumors, which takes 5 to 7 years of clinical evaluation to establish historical and outcome data.
Predictive Oncology enjoys significant competitive advantage. The company already has a vast historical collection of tumors and related data, plus the ability to obtain existing associated outcome data. While others wait for outcome data, Predictive Oncology is in a unique and powerful position, working to deliver the promise of precision medicine to reality. Predictive Oncology already has the clinical data, including how a tumor responded to certain drugs, an in-house bioinformatics AI platform, and only needs to do the tumor sequencing. The significance is underscored by the collaboration with UPMC Magee-Women’s Hospital, designed to reveal which mutations responded to which drug then develop powerful predictive models for future testing and treatment.
Leadership Team
Dr. Carl Schwartz was appointed to Skyline Medical’s board of directors in March 2015 and became interim president and CEO in May 2016. Dr. Schwartz became CEO of Plastics Research Corporation in 1988, leading the company to become the largest manufacturer of structural foam molding products in the U.S. with more than $60 million in revenues and 300 employees by the time he retired in 2001. He holds a bachelor’s degree and DDS degree from the University of Detroit.
CFO Bob Myers has over 30 years of experience in multiple industries focusing on medical device service and manufacturing. He has spent much of his career as a CFO and controller. Myers holds an MBA in Finance from Adelphi University and a BBA in public accounting from Hofstra University.
Gerald Vardzel, President of Helomics, has over 25 years of healthcare executive management experience developing and implementing commercialization strategies and models for technology launches. His Go-To-Market expertise includes equity financing, strategic planning, market intelligence, M&A, and new market development in both start-up and established settings including fortune 500 market leaders. He has developed innovative solutions for both CLIA and FDA regulatory paths defining the delivery chains from discovery to clinical acceptance. Mr. Vardzel also has significant experience designing and implementing sales and marketing programs tailored not only to expand market share, but to empirically assess client satisfaction, strengthen business processes, and maximize profitability. Mr. Vardzel was previously Vice President of Corporate Development and Strategic Initiatives at Global Specimen Solutions. Furthermore, as an executive affiliate to the healthcare industry, he routinely consults for several small-to-mid sized private equity firms advising on, in part, the feasibility of acquisition targets. Mr. Vardzel graduated from the University of Pittsburgh.
Dr. Mark Collins, Chief Information Officer of Helomics, has held multiple executive roles in a variety of discovery, informatics and bioinformatics functions within global pharma, and founded three startup software companies in the machine learning and drug discovery space. In 2001, Dr. Collins worked for Cellomics (now part of Thermo Fisher Scientific), where he played a pivotal role in establishing the High-Content Cell Analysis market, building and commercializing several key informatics and bioinformatics products. After leaving Thermo Fisher, Dr. Collins developed and commercialized informatics solutions for clinical and translational research, specifically in the specimen tracking, omics data management and NGS analysis space, through key roles at BioFortis, Global Specimens Solutions and Genedata. Dr. Collins received his undergraduate degree in Applied Science from the University of Wolverhampton, UK and his Ph.D. in Microbiology from the University of Surrey, UK.
Pressure BioSciences Inc. (PBIO)
Pressure BioSciences Inc. (PBIO) develops, markets and sells proprietary laboratory instrumentation and associated consumables to the life sciences sample preparation market. Sample preparation refers to the wide range of activities that precede most forms of scientific analysis. It is often complex and time-consuming, yet a critical part of scientific research. The market for sample preparation products is currently estimated at $6 billion worldwide.
The Company’s product line can be used to exquisitely control the sample preparation process. It is based on a patented, enabling technology platform called pressure cycling technology (“PCT”). PCT uses alternating cycles of hydrostatic pressure between ambient (14.5 psi) and ultra-high levels (up to 100,000 psi) to safely and reproducibly control critical biological processes, such as the lysis (breakage) of cells, the digestion of proteins, and the inactivation of pathogens.
Pressure BioSciences’ product line is led by its newly released, next-generation Barocycler 2320EXTREME instrument. Named a finalist in the prestigious 2017 R&D Awards (also known as the “Oscars of Innovation”), the Barocycler 2320EXT is already being touted by some key opinion leaders as an essential element of the $1.8 billion U.S. “Cancer Moonshot” program. For example, Professor Phil Robinson, Co-head of the cancer research center of the Children’s Medical Research Institute (Sydney, Australia), said in a recent interview: “We are collecting the whole proteome on 70,000 tumor samples from all classes where complete clinical outcome is known. Due to its unique capabilities, the Barocycler 2320EXT has become a critical part of our program. It is the primary enabler of the high-throughput component of the project. Without this step, our project simply could not be done. In fact, the Barocycler 2320EXT works so well we have just purchased two more.”
Momentum is building when it comes to the potential for using the Company’s unique PCT technology platform. Leading scientists are intrigued by Pressure BioSciences’ approach, which among other attributes, revolutionizes the process of rupturing cells (lysis) for further study, yielding superior biomolecules for investigation. The Company’s technology transcends current methods of breaking open cells, which use chemicals, blades, metal beads, or other damaging and altering methods that can ultimately adversely affect the result for researchers. Pressure BioSciences’ PCT technology utilizes customized, controlled hydrostatic (water) pressure to rupture cells in a chamber, enabling exquisitely customized levels of pressure to optimally break open different types of cells at prescribed pressure levels—something never before accomplished in a commercial setting. Using this pioneering method, the result is a truer, more legitimate sample, which boosts the efficacy of research and the quality of results. The potential impact of this technology on scientific advancement is enormous, enabling research scientists to begin their studies with biological samples of unprecedented integrity, with the potential to improve research outcomes at the earliest, most critical step. PCT can additionally inactivate pathogens (e.g., viruses, bacteria) using hydrostatic pressure, making the samples safer to study—another innovation with astronomical potential for application in a variety of markets.
The Company’s high-pressure instruments for research purposes are marketed throughout the United States, Europe, China and Japan. To date, Pressure BioSciences has installed nearly 300 PCT Systems in over 165 leading academic, government, biotech and pharma laboratories around the world. Its primary applications are in biomarker discovery, forensics, agriculture and pathology. Over 100 scientific papers have been published on the advantages of the PCT platform, which is also being used in the specialized fields of drug discovery and design, bio-therapeutics characterization, soil and plant biology, vaccine development and histology.
Impressive as their biotech business is, there is more to the PBI story. Pressure BioSciences recently received two patents in China for its novel Ultra Shear Technology (UST), a process that has potential in a wide range of industrial applications, including extending the shelf life of some food products and making two insoluble liquids (like oil in water) soluble. Patents have also been filed in many other countries worldwide. UST is a novel technique based on the use of intense shear forces generated from ultra-high-pressure valve discharge.
This important technology has the potential to play a significant role in a number of commercially important areas through its ability to create high-quality, stable nanoemulsions. Scientific studies indicate that improved absorption, higher bioavailability, greater stability, lower surfactant levels and other advantages can be achieved with nanoemulsions – all hugely important factors in the fields of nutraceuticals, cosmetics, pharmaceuticals, and in various medical products. There is an enormous opportunity in the cannabis market, since the technology can potentially reduce oil droplets containing cannabidiol (CBD) to nanoparticles, after which they can be safely suspended in a stable water solution—something many companies have endeavored to achieve without success. Researchers looking for a way to increase the bioavailability of cannabinoids in the body will find this technology a game changer.
The Company’s UST technology also has possibilities in the production of clean label foods, which are currently processed using several innovative methods, including high-pressure treatments (such as Starbucks’ Evolution line of juices). In 2015, the worldwide market for high-pressure processed (HPP) food was estimated at U.S. $10 billion. UST uses ultra-high pressures and certain valves to generate intense shear forces under controlled temperature conditions to produce nanoemulsions, and which also significantly reduces food-borne pathogens. Pressure BioSciences’ initial focus with this technology will be to evaluate UST for the production of high-quality dairy products and beverages.

Prime Harvest Inc., based in San Diego, California, is a technology-focused, full-service cannabis company with horizontally diversified operations spanning various segments of the cannabis value chain, from licensing acquisition and compliance management to direct-to-consumer operations. The company is leveraging a long-term strategy of investing in the growth and scale of licensed assets anchored by the power of data-driven technology to expand its footprint throughout California.
Sustainability is key to Prime Harvest’s corporate vision. The company aims to ensure that the communities it serves capture their fair share of the fruits of the industry’s growth, including financial profit, employment opportunities, environmental enrichment and impactful innovation through R&D and education.
The company’s mission is to appeal to the ethos of the cannabis consumer by setting a new operational standard emphasizing accountability, sustainability and community. With this commitment, Prime Harvest continues to work toward positively affecting millions of lives through the creation of a world-class platform that caters to strengthening the commercial cannabis pipeline.
Jaxx Cannabis
Jaxx Cannabis is the flagship brand in Prime Harvest’s portfolio. Through Jaxx Cannabis, the company aims to use technology to facilitate a true customer-centric culture while enhancing the overall craft cannabis experience. Jaxx features an expertly curated selection of premium products from some of the most respected brands in the thriving California market.
Key values serving as the foundation of Jaxx Cannabis include:
- Creating and nurturing a welcoming culture for all
- Unlocking the true potential of customer value
- Being innovative in uncovering new ways to grow both the company and the industry
- Meeting the wants and needs of consumers to promote profitability
- Remaining accountable for the results of its operations
It is these values that differentiate Prime Harvest and Jaxx Cannabis in the California cannabis sector.
Brand Partnerships
Prime Harvest works diligently to establish strong alliances with complementary brands that are in alignment with its culture and values. Through a combination of deliberate foresight and strategic action, the company seeks to grow existing cannabis brands and continuously discover new, high-potential performers that are primed for long-term success.
These partnerships enhance Prime Harvest’s efforts to transform the world’s cannabis access and bring its consumers high-quality products that are fair for both people and the planet.
Responsibility
Prime Harvest remains committed to the goal of creating a more sustainable environment, now and in the future. Concern for human beings and the environment can be observed in every facet of its operations, including its ongoing R&D activities dedicated to exploring methods of reducing and repurposing waste into composite materials and exploring the potential of the hemp plant for industrial and wellness contributions.
The company is a proud member of the Community Alliance Program, a foundation that seeks to make a difference in local communities by providing financial assistance for educational programs, housing homeless veterans, creating urban farms, and holding local arts initiatives for children and adults. The program also helps explore the natural healing attributes of medical cannabis through research, development, clinical trials, and advocating for the safe access of cannabis to those in need.
Market Overview
Ongoing changes in U.S. state government policies toward cannabis are expected to cause demand for legal marijuana to surge. In addition, the number of indications for which medical marijuana is prescribed continues to increase. These factors are expected to rapidly boost legal sales of cannabis products.
Legal sales across the U.S. hit a record of $17.5 billion in 2020, marking an increase of 46% over 2019, according to Forbes. This strong growth is expected to continue. According to a Grand View Research report, the global legal marijuana market is forecast to grow at a CAGR of 26.7 percent from 2021 to 2028.
California – Prime Harvest’s home state – has consistently led the pack in terms of U.S. cannabis sales. The Motley Fool pegged cannabis spending in the Golden State at $3.8 billion in 2020, more than doubling the second state on its list.
Leadership Team
The Prime Harvest team is composed of true experts in their respective fields focused on building a world-class organization capable of driving the cannabis industry and movement forward.
E. Duane Alexander is the company’s Founder and CEO. He brings to the team more than 25 years of real-world, hands-on cannabis retail, marketing and commercial operations experience. Mr. Alexander has championed 40+ cannabis license applications throughout the western U.S. to date.
John Wilczak is the COO of Prime Harvest. He has 30+ years of executive management, strategy development & configuration experience with GE, pharmaceutical and agriculture companies. Mr. Wilczak is a Brown & Columbia MBA with vast knowledge of technology driven intellectual properties.
Andrea Jenson is the Chief Financial Officer of Prime Harvest. As CFO, she is responsible for all the company’s financial functions, including accounting, corporate finance and investor relations. Her career spans more than 20 years of varied experience in financial management, business leadership and financial strategy.
John Kazanjian is the VP of Business Development of Prime Harvest. He has worked over 40 years in business operations, brand marketing, sales and investor/lender communications. Mr. Kazanjian earned his B.S. from Rutgers University and his MBA from Harvard University.
Johann Balbuena is the Chief Marketing Officer of Prime Harvest. She has more than six years of experience in California cannabis licensing acquisition and compliance management. Ms. Balbuena has led multimedia production and content marketing efforts for the likes of the Social Club TV app, The Emerald Cup, High Times, Weedmaps and Synergy.
Processa Pharmaceuticals Inc. (NASDAQ: PCSA)
Processa Pharmaceuticals Inc. (NASDAQ: PCSA) aims to develop products where existing clinical evidence of efficacy already exists in unmet medical need conditions. In support of this goal, the company has assembled an unparalleled management team, board of directors and product development team featuring experts in developing drug products, from IND-enabling studies to NDA submission. In total, the team’s combined scientific, development and regulatory experience has resulted in more than 30 drug approvals by the U.S. Food and Drug Administration (FDA) and more than 100 meetings with the FDA while working on more than 50 drug development programs, including drug products targeted to orphan disease and unmet medical need conditions.
Headquartered in Hanover, Maryland, Processa has built a pipeline of drugs which already have some proof-of-concept clinical data supporting clinical use in their selected indications.
Development Pipeline
The Processa process focuses on the advancement of drugs that are ready for clinical development or have minimal pre-IND enabling studies to complete. More specifically, Processa:
- Acquires drugs that already have some clinical data to support the targeted treatment – whether it be the drug itself, an analog of the drug or a drug with similar pharmacological targets;
- Navigates through the FDA, collaborating with the reviewers to define a complete development program; and
- Develops each drug over the course of 2-5 years, out-licensing the drug either just prior to pivotal study after Phase 2b or after the completion of the pivotal study.
Processa’s current development pipeline features multiple drug candidates, including PCS499 and PCS100. The company has also announced three additional licensing agreements since June 2020, further bolstering its clinical efforts. Each drug is briefly described below.
PCS6422
On August 27, 2020, Processa announced its entry into a contingent precedent exclusive licensing agreement with Elion Oncology Inc. to develop, manufacture and commercialize eniluracil (PCS6422) globally. PCS6422 is an oral drug to be administered with fluoropyrimidine cancer drugs (e.g., capecitabine, 5-FU) to decrease the breakdown of the cancer drug to inactive metabolites or metabolites that are known to cause unwanted side effects and to increase the anti-cancer related metabolites.
An IND for a Phase 1B study was cleared by the FDA in May 2020. The study will evaluate the safety and tolerability of several dose combinations of PCS6422 and capecitabine in advanced GI tumor patients. Processa intends to enroll the first patient in 1H2021, obtain interim results, and have a final report completed in 2H2022.
“Having worked on 5-FU and other cancer agents in the past, adding PCS6422 to our pipeline and expanding our involvement in oncology was an easy decision given the significant impact that PCS6422 may have on improving the efficacy and safety of capecitabine or other fluoropyrimidines,” CEO Dr. David Young said of the agreement.
PCS499
PCS499 as a potential treatment for necrobiosis lipoidica (“NL”) was first presented to the FDA in a pre-IND meeting in 2018. In 2019, it was the subject of an IND submission and a promising Phase 2 safety study. On March 30, 2020, Processa announced a successful meeting with the FDA regarding the design and execution of the next clinical study to evaluate the ability of PCS499 to completely close ulcers in patients with NL.
“We are pleased with the outcome of the FDA meeting and the feedback we received from the FDA. We believe that the results from our completed Phase 2 trial in NL patients, especially those with more severe ulcerated forms of NL, are encouraging and we appreciate the guidance provided by the FDA regarding our next clinical trial and the requirements to support our NDA submission,” Dr. David Young, CEO of Processa, stated in the news release.
NL is a chronic, disfiguring condition affecting the skin and tissue under the skin, typically on the lower extremities, with no currently FDA-approved treatments. More severe complications can occur, such as deep tissue infections and osteonecrosis, threatening the life of the limb. Approximately 22,500 – 55,500 people in the United States and more than 150,000 – 400,000 people worldwide are affected by the ulcerated form of NL.
YH12852
On August 20, 2020, Processa announced its entry into an agreement with Yuhan Corporation, a South Korean firm, to license YH12852, a small molecule drug in development for the treatment of functional gastrointestinal (GI) disorders. Under the terms of the agreement, Processa will acquire the rights to a portfolio of patents with an exclusive license to develop, manufacture and commercialize YH12852 globally, excluding South Korea.
YH12852 is a novel, potent and highly selective 5-hydroxytryptamine 4 (5-HT4) receptor agonist. Other 5-HT receptor agonists with less 5-HT4 selectivity have been shown to successfully treat GI mobility disorders such as chronic constipation, constipation-predominant irritable bowel syndrome, functional dyspepsia and gastroparesis. The less selective 5-HT4 agonists, such as cisapride, have been removed from the market because of the cardiovascular side effects associated with the drugs binding to other receptors, especially 5-HT receptors other than 5-HT4.
CEO Dr. David Young called the agreement “further evidence of Processa’s commitment to seek out novel treatments for unmet medical conditions.” Processa intends to meet with the FDA in early 2021 to further define the clinical development program. In 2021, Processa expects to initiate a Phase 2 trial in a functional GI motility-related disorder that that needs better therapeutic options, such as postoperative ileus and opioid-induced constipation.
ATT-11T
On June 1, 2020, Processa announced its entry into a licensing agreement with Aposense Ltd. for the patent rights and know-how to develop and commercialize ATT-11T, a next generation irinotecan cancer drug. In the release, CEO Dr. David Young noted that the licensing deal fit with Processa’s strategy to “continue to bring innovative products to patients with an unmet medical need condition.”
ATT-11T is a novel lipophilic anti-cancer pro-drug that is being developed for the treatment of the same solid tumors as prescribed for irinotecan. This pro-drug is a conjugate of a specific proprietary Aposense molecule connected to SN-38, the active metabolite of irinotecan. The proprietary Aposense molecule on ATT-11T allows ATT-11T to bind to cell membranes to form an inactive pro-drug depot on the cell, with SN-38 preferentially accumulating in the membrane of tumors cells and the tumor core. This unique characteristic is expected to make the therapeutic window of ATT-11T wider than irinotecan, such that the anti-tumor effect of ATT-11T will occur at a much lower dose than irinotecan with a milder adverse effect profile than irinotecan. The wider therapeutic window will likely lead to more patients responding with less side effects when on ATT-11T compared to irinotecan.
The ATT-11T licensing agreement is conditioned upon Processa’s closing of a satisfactory financing round and the listing of the company’s shares on the Nasdaq or NYSE, among other conditions.
PCS100
On September 3, 2020, Processa announced its entry into an exclusive worldwide license agreement with Akashi Therapeutics to develop and commercialization Akashi’s lead drug, HT-100. Rebranded PCS100, the candidate is an anti-fibrotic, anti-inflammatory drug demonstrated to have some clinical anti-fibrotic effect in children. Processa intends to develop PCS100 first in rare adult fibrotic related diseases such as focal segmental glomerulosclerosis (FSGS), idiopathic pulmonary fibrosis (IPF) or Scleroderma, where there are still few therapeutic options.
Management Team
David Young, Pharm.D., Ph.D. is the CEO and founder of Processa. He has over 30 years of pharmaceutical research, drug development and corporate experience. Young has served in leadership roles with a number of pharmaceutical firms throughout his career, including serving as founder and CEO of Promet Therapeutics LLC since 2015 and as Chief Scientific Officer of Questcor Pharmaceuticals from 2009 to 2014. At Questcor, he was responsible for working with the FDA on modernizing the Acthar Gel label and for obtaining FDA approval in infantile spasms. In total, Young has met with the FDA more than 100 times on more than 50 drug products and has been a key team member on more than 30 NDA/supplemental NDA approvals.
Sian Bigora, Pharm.D., is Processa’s Chief Development Officer and founder. She has over 20 years of pharmaceutical research, regulatory strategy and drug development experience, working closely with Young. Prior to joining Processa, Bigora served as Co-Founder, Director and Chief Development Officer at Promet Therapeutics LLC and as Vice President of Regulatory Affairs at Questcor Pharmaceuticals from 2009 to 2015, where she led efforts to modernize the Acthar Gel label and obtain FDA approval in infantile spasms – events which were of material importance to Questcor’s subsequent success.
Patrick Lin is Chief Business & Strategy Officer and founder of Processa. He has over 20 years of financing and investing experience in the biopharma sector. Prior to joining Processa, Lin served as Co-Founder and Chairman of Promet Therapeutics LLC. He is also founder and managing partner of Primarius Capital, a family office that manages public and private investments focused on small capitalization companies.
James Stanker has served as CFO of Processa since 2018. He has over 30 years of financial and executive leadership experience in the areas of accounting principles and audit standards, regulatory reporting, and fiscal management and strategy. He served in a financial leadership role as an audit partner at Grant Thornton from February 2000 until his retirement in August 2016, where he was responsible for managing audit quality in the Atlantic Coast market territory.
Wendy Guy is the Chief Administrative Officer and founder of Processa. She has more than two decades of experience in business operations, having worked closely with Young over the last 18 years in corporate management and operations, HR and finance. Prior to joining Processa, she was Co-Founder, Director and Chief Administrative Officer of Promet Therapeutics LLC and Senior Manager, Business Operation over the Maryland office for Questcor Pharmaceuticals.
QSAM Biosciences Inc. (OTCQB: QSAM)
QSAM Biosciences Inc. (OTCQB: QSAM) is a clinical stage biotechnology company focused on bringing to market targeted therapeutic radiopharmaceuticals. The company is committed to advancing the fight against cancer through the discovery, development and delivery of effective treatment options for adult and pediatric patients.
QSAM Biosciences was founded in 2020 by Executive Chairman Dr. C. Richard Piazza and CEO Douglas Baum. It is headquartered in Austin, Texas.
CycloSam®
CycloSam®, QSAM Biosciences’ initial technology, is a clinical-stage bone targeting radiopharmaceutical invented by world-renowned scientists at IsoTherapeutics Group LLC. By leveraging a patented, low specific activity form of Samarium-153 (resulting in far less undesirable europium impurity) and what management believes to be a superior chelating agent in DOTMP, CycloSam is designed to selectively target sites of high bone mineral turnover to deliver a prescribed tumor-killing dose of radiation to the bone tumor sites while minimizing radiation exposure to nearby healthy tissue. These parameters are currently being tested in an FDA-cleared clinical trial.
CycloSam® has been shown in laboratory testing to cause significantly less (30x less) buildup of long-lived radionuclidic impurities than prior FDA-approved drugs, which management believes will enable the ability to safely administer therapeutic doses via higher and multiple-dose regimens and effectively expand its potential clinical utility to therapeutic uses in areas of high unmet medical needs.
The indications for CycloSam® currently being evaluated by QSAM Biosciences include:
- Metastatic Bone Cancers – On April 28, 2022, QSAM Biosciences announced that the first patient had commenced treatment in its clinical trial evaluating CycloSam in patients with metastatic bone cancer. As noted in the release, the study is a Phase 1 open-label, dose-escalation trial to evaluate the safety, tolerability, dosimetry, and preliminary efficacy of CycloSam®.
- Pediatric Osteosarcoma/Ewing’s Sarcoma – On February 2, 2022, the company announced that the U.S. FDA has granted Rare Pediatric Disease Designation to CycloSam for the treatment of osteosarcoma. Combined with a previously granted orphan drug designation for osteosarcoma received in 2021, this milestone “may allow QSAM to potentially bring CycloSam® to market more rapidly through additional incentives and eligibilities,” according to CEO Douglas Baum.
- Bone Marrow Ablation – In a 2020 single patient Investigational New Drug (IND) study, an investigator concluded that high-dose CycloSam® can be administered safely to ablate bone marrow in advance of a stem cell transplant with no apparent renal toxicity and no unexpected adverse events attributable to the drug.
QSAM Biosciences’ preclinical and clinical development pipeline is supported by a strong IP portfolio. The company has secured 14 patents across three distinct patent families spanning the U.S., Japan, Canada and the European Union.
Market Outlook
Through its ongoing development of CycloSam®, QSAM Biosciences is targeting multiple large and underserved market opportunities. According to the American Cancer Society, roughly 400,000 new cases of malignant bone metastasis are diagnosed annually in the U.S. alone. Additionally, QSAM will pursue indications for osteosarcoma and Ewing’s sarcoma that are the most common primary malignancies of bone tissues in children.
Despite this pressing need, the current standard of care for bone cancer is aggressive and suboptimal, leading to marginal success with significant side effects and poor long-term survival prognosis. As a result, QSAM Biosciences estimates a sizable market opportunity for its development pipeline.
- Bone Metastasis has an estimated total addressable market of $20 billion in the U.S. based on total new cases and comparable drug pricing.
- Osteosarcoma/Ewing’s Sarcoma have a total addressable market of roughly $125 million in the U.S. based on approximately 1,000 new cases in 2021.
- The total addressable market for Bone Marrow Ablation is projected at $1 billion, with an estimated 32,000 procedures completed annually.
The company anticipates that the ability to administer CycloSam® for higher and multiple-dose regimens may expand its clinical utility for therapeutic uses in additional areas of high unmet medical needs.
Management Team
QSAM Biosciences is led by an experienced management team and board with an extensive record of FDA approvals, big pharma partnerships and M&A transactions.
Dr. C. Richard Piazza is the Executive Chairman of QSAM Biosciences. Since 2017, he has also served as President and CEO of IGL Pharma Inc., the licensor of CycloSam®, and as a consultant to IsoTherapeutics Group LLC, the inventors of the technology. Dr. Piazza also currently serves on the board of directors of NovaScan LLC, a privately held cancer detection and diagnostics company. He has more than 48 years of health care experience in both medical devices and pharmaceutical/biotech and has led several technology companies to market success, including numerous FDA approvals in both sectors. Dr. Piazza obtained a BS in Economics and a BS in Speech Pathology from the State University of New York and an MA & PhD in Economics from the University of Buffalo and Leeds University.
Douglas R. Baum is the company’s CEO and Director. He brings to QSAM Biosciences over 30 years of experience in the bioscience and biotech industries, including development, FDA/EMA approval and commercialization of multiple drugs and medical devices. Mr. Baum has overseen 15 product approvals through the FDA and EMA and raised over $85 million in capital to fund breakthrough technologies. From 2017 to 2020, he consulted with multiple medical schools and biotech and pharmaceutical companies, and, from 2012 to 2017, he served as President, Chief Executive Officer and Director of Xeris Pharmaceuticals Inc. Mr. Baum holds a Master of Science in Technology Commercialization and a BBA in International Business and Marketing from the University of Texas.
Adam King is the CFO of QSAM Biosciences. He is also the Founder and CEO of King Consulting Group, where he provides a range of financial and reporting services for clients. Before founding King Consulting Group in January 2021, Mr. King was the CFO for Netsertive, a venture-backed digital marketing company. From 2016 to 2018, he was the Office Managing Audit Director for BDO’s Greenville, South Carolina, office, in addition to serving as Audit Director in Raleigh, North Carolina, and Boston, Massachusetts. While at BDO, Mr. King worked with various clients, from tech and life science start-ups to billion-dollar publicly traded companies. He holds a Bachelor of Science in Accounting from Elon University and is a CPA in Raleigh, North Carolina.
Red White & Bloom Brands Inc. (CSE: RWB) (OTCQX: RWBYF)
Red White & Bloom Brands Inc. (CSE: RWB) (OTCQX: RWBYF) is a torchbearer blazing a new frontier in American cannabis by adhering to the highest ethical, manufacturing, educational, branding and employment standards available in the industry.
Red White & Bloom is a super state operator, leveraging a sizable footprint to dominate the areas in which it operates. CEO Brad Rogers and other management members have seen the struggles of multi-state operators who have spread themselves too thin, which is why Red White & Bloom is intent on dominating each state it enters before expanding further.
Although targeting individual states in the United States, the company is headquartered in Toronto, Canada. Red White & Bloom was established after privately held MichiCann Medical Inc. merged with publicly traded Tidal Royalty in 2019.
Brands
Red White & Bloom has entered strategic brand acquisitions and partnerships aimed at helping the company expand its presence and position as one of the largest players in the United States cannabis market. Red White & Bloom is always diligently searching for brands to acquire that will provide additional value to the company and expand its national footprint.
The company’s current brand portfolio includes:
- Platinum Premium Cannabis Products (PV): Platinum uses innovative thinking, honesty and responsibility to remain at the forefront of the cannabis industry. PV holds itself and its partners to the highest standards, providing clean and safe CBD and THC products. In the company’s press release dated January 13, 2021, it reported system-wide sales of Platinum-branded products exceeding $2.8 million for the first week of January alone.
- High Times®: In June 2020, the company acquired the licensing rights and branding of High Times dispensaries and High Times cannabis-based CBD and THC products in Michigan, Illinois and Florida. The company also acquired branding of High Times hemp derived CBD products nationally in the United States carrying the Culture® brand.
- Mid-American Growers: Mid-American began as a family operation in 1971 in Granville, Illinois. The original 8-acre greenhouse has expanded to a 3.6-million-square-foot, state-of-the-art technology and science facility under glass. Mid-American’s product offerings include its CBD Icy Relief Salve, CBD Icy Relief Roll-on and CBD Gummies.
Retail Focus
Red White & Bloom is working to establish a significant retail presence across multiple jurisdictions. In Michigan, the company is invested in and has the rights to acquire (subject to regulatory approvals) a licensed operator that controls the assets of 18 dispensary locations throughout the state. Red White & Bloom is also pursuing opportunities in Florida aimed at making its proposed retail footprint compelling and attractive to the majority of cannabis consumers within each state.
Cultivation
Red White & Bloom is focused on standardization and quality, with everything guided by a relentless commitment to the highest standards. The company acquired a 3.6-million-square-foot standardized facility dedicated to helping it achieve premium value for the products it intends to cultivate.
As it continues to expand, the company remains committed to the practices that have guided its success in the past, including:
- A top-down approach to cultivation developed under the guidance of PhDs with expertise in growing principles, SOPs and, most importantly, the science behind it all.
- Commitment to exceeding the requirement of the states in which it operates. The company cut its teeth under the world’s first national cannabis purity regime – a regime that most new markets use as a benchmark – so quality is in its DNA.
- Science-driven production methods supported by automated, perpetual, standardized operations that enable craft cannabis-like quality at an industrial scale.
Footprint
Assuming completion of the currently proposed investments and acquisitions, Red White & Bloom will be among the cannabis market’s largest companies, joining the ranks of a select few multi-state operators dominating the industry. Red White & Bloom currently has assets (closed and in closing stages) in Michigan, Illinois, Florida, California, Oklahoma and Massachusetts.
The company’s strategic acquisition and super state operator model, combined with its commitment to top-quality product and service, position it to become a leading player in the North American cannabis market.
When evaluated beside competitors in the cannabis space, Red White & Bloom boasts an extremely attractive valuation. While large cap cannabis firms serving North American markets averaged enterprise-value-to-EBITDA multiples of 14.9x as of December 2020, Red White & Bloom’s enterprise multiple was just 3.4x, as noted in the company’s latest investor deck.
In 2020, the cannabis market worldwide was valued at $24.6 billion. This amount is expected to expand at a CAGR of 14.3% from 2021 to 2028, resulting in a market size of $84 billion in 2028 (https://nnw.fm/f09ZL). Of the 2020 valuation, the largest revenue share (91.1%) was attributed to North American consumers (https://nnw.fm/vObW6).
Management Team
Brad Rogers is the CEO and Executive Chair of Red White & Bloom. He is a visionary for the future of cannabis and CBD products in the United States market, with a proven track record of building successful and profitable businesses in the rapidly expanding and new economic sector. Mr. Rogers was a part of the team that built one of the first commercially scaled production facilities in the world for medicinal cannabis. He also served as President for one of the leading licensed producers in Canada. Both of his ventures were successful, with a combined market cap of $2 billion.
Michael Marchese is the company’s Co-Founder and Marketing Advisor. He has played a crucial role in its development and organization, overseeing capital raises, acquisition strategy and brand identity. Mr. Marchese has a strong reputation and presence in the cannabis industry. He also co-founded and directed the branding of Aleafia Health Inc., which he continues to counsel. Through his branded company, Marchese Design, he has served as a highly trusted counselor to top-level execs, including C-Suite level employees, offering insights into the process of creating, building and maintaining brand identities.
Theo van der Linde is the CFO and Director of Red White & Bloom. He is a Chartered Accountant with 20 years of experience in finance, administration and public accounting. The experience he has acquired spans multiple industries, including mining, oil & gas, financial services, retail and manufacturing. For the last nine years, he has primarily focused his career on the mining industry, working with junior exploration and producing mining companies at various stages of growth in several jurisdictions. Mr. van der Linde is also the current President of Executive Management Solutions Ltd.

REZYFi, Inc. is a cannabis mortgage bank servicing the needs of both traditional and non-traditional consumers and businesses. Its target markets include licensed and permitted cannabis companies, owners of real estate who lease to cannabis companies, and companies and individual homeowners seeking a variety of real estate-related first and additional mortgage-based financing and project-specific financings, such as solar installations and real estate development projects.
Headquartered in Miami, Florida, REZYFi operates through two wholly owned subsidiaries – REZYFi Lending, which primarily addresses emerging real estate-related financing opportunities, and ResMac Inc., the company’s traditional mortgage origination, correspondent and servicing operation. REZYFi is currently licensed in 34 U.S. states, with plans to expand to all remaining states later this year.
REZYFi is positioned as one of first cannabis mortgage bankers in the U.S., while most traditional lenders are still reticent to serve the state-licensed cannabis industry.
Operations
REZYFi Lending
REZYFi Lending leverages a wide network to offer options such as 15- and 30-year fixed-rate loans, FHA loans, VA loans, reverse mortgages, jumbo loans and adjustable-rate mortgages.
Looking ahead, the company expects increased funding in marketing and loan agents to drive significant origination growth over the next two years, further supported by the planned launch of a high-margin cannabis division later this year.
ResMac Inc.
ResMac has been in operation for 13 years, having closed more than 20,000 loans for more than 15,000 clients. The company expects to accumulate $285 million in retail origination in 2023, alongside $250 million in wholesale origination for the same period. ResMac is further targeting $600 million in origination through its mortgage correspondent operations for 2023.
Through its ResMac subsidiary, REZYFi operates as a direct lender and originator of residential mortgages, with active mortgage correspondent and mortgage servicing operations. Through its correspondent segment, ResMac primarily purchases and aggregates residential mortgages from trusted third-party originators.
The company intends to harvest the database of customers within its mortgage servicing operations as an essential source of additional growth, especially relative to the new alternative residential loan programs being offered.
Corporate Strengths
- Experience – REZYFi is led by a seasoned management team with significant expertise spanning a wide range of real estate and financing subsectors. The team also has extensive experience in the cannabis and hemp marketplace, which the company intends to leverage as it navigates the changing landscape of the cannabis industry while sourcing the best opportunities in the sector.
- Network of Independent Brokers – Over the past five years, REZYFi has developed an extensive network of independent mortgage-related brokers and licensed loan officers. The company is currently training the network members on its new service offerings, with many already launching sales efforts. REZYFi believes this network will be a vital asset moving forward as other firms in the sector terminate relationships in the face of slowing mortgage business in a rising interest rate environment.
- Proprietary Technology – REZYFi has invested heavily in designing, building and implementing proprietary automated/machine learning technology to shorten loan processing timeframes and increase efficiencies, allowing it to operate its legacy business at staffing levels meaningfully below those of its competitors.
Market Overview
REZYFi’s diversified approach to the real estate lending sector positions it to capitalize on growth in multiple verticals in the years to come.
In the first quarter of 2022, lenders issued 2.71 million residential loans, with the average balance for a first mortgage climbing to a record high of $298,324 in 2021, according to the Mortgage Bankers Association. This trend is expected to continue, with Freddie Mac forecasting a 10.4 percent increase in home prices in 2022 and a 5.0 percent bump in 2023. Growth prospects in the cannabis industry paint a similar picture.
The National Association of Realtors® issued a report in April 2021 examining the correlation between cannabis legalization and real estate demand. In states where prescription and recreational cannabis use is legal, more than a third of surveyed agents reported an increase in demand for warehouses. Likewise, 23 percent of those surveyed reported an increase in demand for storefronts, and 28 percent observed increased demand for land. As other states look to join the 19 that have embraced full cannabis legalization, this rising demand could create an opportunity for REZYFi’s cannabis-focused initiatives.
In total, an analysis by market research firm Business Research Insights projects the global loan servicing market to reach a value of nearly $1.5 billion by 2028, up from $680.8 million in 2021. Those figures represent a CAGR of 11.0 percent during the forecast period of 2022-2028.
Management Team
John Vu, Esq., is CEO of REZYFi, Inc. He has more than two decades of experience in the mortgage and commercial banking industry. He has filled many senior and executive management positions in high-producing mortgage banks, including C-level assignments. He has also served as general counsel for a nationally associated commercial bank. Mr. Vu brings considerable cannabis industry expertise to REZYFi. He has served as a corporate attorney to multiple cannabis cultivators, manufacturers and retailers.
Ji Ji Zhang, Esq., is CFO of REZYFi, Inc. He is a multifaceted entrepreneur who owns a law firm, a portfolio of hotels and a high-producing mortgage bank. Mr. Zhang is also an investor in the development of a cannabis business park. He brings more than five years of experience in mortgage banking to REZYFi, having developed Freddie Mac and HUD licenses and amassed a managed portfolio valued at over $300 million.
Kevin Heckemeyer is President of REZYFi, Inc. He has more than 25 years of experience in mortgage banking. He has built and sold several high producing mortgage businesses. In his current roles with ResMac, he is responsible for production and operations.
Spencer Dang is Chief Credit Officer of REZYFi, Inc. He has more than a decade of experience in mortgage operations. He is a direct endorsement underwriter for HUD and has specialized in non-QM underwriting. Under his watch as an underwriter, he has never had a single repurchase.
RYAH Group Inc. (CSE: RYAH)
RYAH Group Inc. (CSE: RYAH) is a leading digital health care analytics and technology company with a mission to advance the world’s transition to remote-health solutions and data analytics in patient treatments. Through the company’s IoT dose-measuring devices and AI analytics, RYAH is reshaping understanding of the value of devices combined with data, to positively impact the future treatment of patients for various medical conditions.
The company is a leading developer of dose-measuring IoT devices connected with its turn-key platform designed to aggregate and correlate HIPPA-compliant data, suitable to all participants in the patient treatment cycle. The company also specializes in customized, fully integrated, mobile applications and APIs, specifically designed to meet the needs of clinics, clinical trials, government and university research centers, for experimentation and treatment validation – significantly reducing variations in patient-related trials. RYAH unlocks data in the complete therapeutic plant lifecycle – from seed to consumption.
Since it began developing and commercializing its smart inhaler solution in 2018, the company has evolved a complete IoT device and data analytics platform that includes multiple delivery mechanisms, designed to capture anonymous patient dosing and feedback, combined with detailed strain analytics, enabling customized dosing regiments. The company has secured numerous partnerships across the globe, including establishing a footprint in the UK, USA, Australia and Canada, and it has closed several deals in the European Union, as well. The company’s Smart-Inhaler has been selected as the dose-measurement, dose-control and data analytics platform for a UK pain management study and one of the world’s most ambitious and largest clinical trials ever to be conducted in cannabis.
Product Portfolio
The company’s current portfolio incorporates an ecosystem of IoT products, each consisting of three elements: the device, the medicine-carrying component and the mobile application. The product line currently includes a Smart Dry-Herb Dose-Measuring Inhaler in the commercial stage, a Smart Transdermal Patch in the production stage and a Smart Liquid Dispensing Pen in the prototype stage.
RYAH Smart-Inhaler
The RYAH Inhaler is the first dry-herb inhaler that allows users to track and control how much is inhaled, providing consistent and predictable results. This inhaler connects with the RYAH Health App, which features stat-tracking and presets for temperatures and dosages, all of which can be customized to individual needs and doctor recommendations, as well as a post-session review mechanism that allows the collection of session data and feedback for further efficacy analysis for customized dosing capabilities.
RYAH’s proprietary stainless-steel cartridges for the inhaler use QR technology that contains lab testing and grower information pertaining to the specific strain, thereby mitigating elicit product use and enabling completely transparent remote medicinal analytics, from seed to consumption.
In addition, the RYAH Cartridges provide a unique closed-loop recurring revenue opportunity for the company, as the RYAH Inhaler only works with this type of proprietary cartridges that licensed partners fill with medicine. The partners benefit from all the back-end data, providing them access to consumption habits, statistics and other data on patient preferences.
RYAH Smart-Patch
The RYAH Smart Transdermal Patch is a lightweight, reusable, mobile-controlled patch used for site-specific therapies. The Patch is an Electronic Topical Delivery Patch system intended for recommendation and administration by pain relief professionals and physical and occupational therapists. The patch data and the heating element is completely IoT and controlled by RYAH’s proprietary smartphone applications, which allows scheduling and ‘boosting’ medicine release, on-demand.
RYAH Smart-Pen
The RYAH Pen is an app-controlled liquid dispenser designed to provide a precise mix of up to three medicine components to create an ‘entourage effect’, enabling customized, wide-spectrum recommendation opportunities by licensed clinicians. The Smart-Pen will feature cartridges that contain CBD, THC and other isolates such as flavonoids or vitamins, or other solutions. There is a built-in mechanism designed to control usage based on recommended dosing schedules.
RYAH MD
RYAH MD serves as a remote and interactive patient-doctor collaboration and dosing administration platform. Doctors can remotely set dosage amounts for their patients, creating digital prescriptions for the RYAH IoT devices and tracking patient usage in real-time. RYAH MD offers features that include real-time monitoring, appointment booking, doctor-patient video calls and science-based strain recommendations, as well as promoting a better understanding of the effects and benefits of those recommendations among patients. Information is gathered from all of the RYAH devices.
PotBot App
The PotBot App is a medical cannabis education mobile application that leverages patented AI technology to capture structured and unstructured data to assist patients in learning about various treatments in plant-medicine based on their efficacy goals. The PotBot App is currently one of the top-rated medical cannabis educational mobile applications on the Apple App Store in the United States, with over 300,000 downloads.
Through the combination of peer-reviewed and empirical data, the PotBot App provides detailed information on the targeted and tested cannabinoid levels and associated strains from cannabis patients. The result is personalized and driven by data to inform patients of potential product matches associated with similar ailments and efficacy goals.
Market Outlook
RYAH holds a unique position in the $100.3 billion medical plant market, with the potential to capture and capitalize on growth opportunities made available by both the IoT and Data Intelligence sectors.
In 2018, the global IoT market was valued at $212.1 billion, and it is expected to grow exponentially to $1.3 trillion by 2026, registering a CAGR of 25.68%, according to Verified Market Research (https://ibn.fm/XtkPZ).
Management Team
Dr. Boris Goldstein, Ph.D., is the founder and Chairman of RYAH Group. He is a seasoned entrepreneur, investment banker and venture capitalist. He started his career as the founder of Software House HT, which grew into a worldwide corporation with over 40 offices in 17 countries. Since then, Goldstein has founded and served on the boards of directors and advisory boards for numerous companies in Silicon Valley and Silicon Alley. Goldstein brings experience in fundamental research, investment and technology, authoring multiple patents and books.
Gregory Wagner, MBA, is Chief Executive Officer and Director of RYAH Group. He has over 20 years of experience in global financial markets and entrepreneurship. Wagner has held executive roles in the United States and London. He has co-founded and built several startups from the ground up. His current licensures and degrees include FINRA Series 7, 63, 24 and 55, as well as an MBA from Fordham University. Wagner received a Certification in Innovation and Strategy from Harvard University.
Sanwire Corp. (SNWR)
Sanwire Corp. (SNWR) is a diversified company currently focused on technologies for the music industry. The company specializes in locating unique opportunities in fragmented markets and implementing its aggregated technologies to consolidate distinct services into unified platforms of delivery. Sanwire is currently focusing these efforts on advanced entertainment technologies.
Founded in 1997 and based out of Las Vegas, Nevada, Sanwire has operated and sold several subsidiaries as it has worked in various industry segments, including Sanwire Software Inc., Bullmoose Mines Ltd. and Squeeze Report Inc. Currently, there are two new holdings that were added to the company’s portfolio through two recent acquisitions, including Intercept Music Inc. in March 2020 and the Art is War Record Label in June 2020.
Intercept Music Inc. – Artist-Focused Services
Intercept Music Inc. is an entertainment technology company offering a unique suite of artist-focused services that are specifically designed to meet the needs of recording artists. Intercept’s proprietary online platform is dedicated to helping millions of global independent artists effectively promote their music and distribute it worldwide to hundreds of digital stores and every major streaming platform, including Spotify, Apple Music, Amazon Music, Pandora and Google Music.
With Intercept Music, recording artists have all the tools needed to market, promote and sell their music online and through social media. Comprehensive reporting allows artists to track the fan response to their releases, all the way down to individual music tracks.
There are three foundations of Intercept Music’s product offering:
- Its music distribution platform that is well augmented via the company’s partnership with InGrooves, a wholly owned subsidiary of Universal Music, which is arguably one of the largest music companies in the world.
- Its social media system, which is tailored to work the way artists use social media to promote their music and engage with their fans. The scheduling system integrates artists’ profiles across multiple social networking sites (Facebook, Twitter, Instagram and YouTube) to facilitate new audience sampling, fan development and the ability for music to be previewed and purchased.
- The third is represented by the team of developers that brings a unique combination of deep technical expertise (in products like Skype), a team of well-accomplished executives and what the company calls Brand Ambassadors – senior reps from multiple genres who have helped artists earn over 100 Grammys.
Intercept Music is the confluence of technology and this music expertise.
The company currently markets three plans to its clients, with each offering different distribution and royalty options, as well as various marketing and reporting options. The plans are described below:
- Intercept Distro is a basic plan for self-service music distribution with royalty collection. Artists keep 100% of the royalties while receiving unlimited releases and full analytics with reporting.
- Intercept Artist includes all of the benefits of the basic Distro plan with added emphasis on social marketing and distribution for emerging artists. With this plan, artists receive scheduled and ad-hoc posting, social media reporting, reusable content libraries and access to other valuable features.
- Intercept PLUS is available by invite only and is for established artists looking for a complete suite of marketing, distribution and monetization services. The PLUS plan includes everything available through the Distro and Artist plans, as well as offering a dedicated service representative, a branded online store, on-demand merchandise, additional marketing, YouTube monetization and other pro features.
Intercept PLUS is the flagship plan. Artists of this caliber often do $3-$10k/month in merchandise sales alone, at 50%+ profit. Intercept is responsible for marketing to the fan base through its social media system and shares in the profits generated. The stores are managed by intercept so both top-line revenues and bottom-line profits flow through Intercept.
Intercept Music has partnered with Ingrooves Music Group, the largest online music distribution company in the world, for worldwide distribution to streaming services and leading stores. Completing more than 50 billion transactions weekly across over 150 countries, Ingrooves supplies music to leading streaming music platforms and lists some of the world’s largest and most reputable music labels among its clients. The partnership allows Intercept Music and its clients to reach a much wider audience and start earning revenue as soon as possible by leveraging Ingrooves’ quality control systems and direct relationships with leading music streaming services.
Physical Distribution Options for Intercept Music Clients
In a press release on June 25, 2020, Intercept Music announced that it would be offering artists physical distribution through major retailers such as Amazon, FYE and Walmart (http://nnw.fm/NSrbE). The physical distribution will consist of CDs and vinyl and will serve as a supplement to the online streaming platform access provided by the company to represented artists.
“In the current climate, artists can’t play shows or otherwise engage in public at all, so they’re focusing on all other opportunities to bring in revenue,” Intercept Music President Tod Turner stated in a news release. “Our only priority is to help artists monetize music in every way, and with physical distribution added to the mix, we’re leaving no stone unturned in helping artists to earn money from their creative output.”
Creation of Preferred Stock
On June 29, 2020, Sanwire CEO Christopher Whitcomb announced that the company would be filing certificates of designation with the Nevada Secretary of State for its Series A, B and C preferred stock (http://nnw.fm/svrQt).
Speaking about this designation in a news release, Whitcomb stated, “Our paramount goal is to maintain a balanced approach between future investments and shareholder value while minimizing shareholder dilution. The effective utilization of preferred stock ensures our company can grow with the least amount of shareholder dilution.”
Sanwire is leveraging a multi-dimensional strategy that includes additional acquisitions, attracting investors and enhancing the current balance sheet while minimizing dilution for shareholders. A primary goal of these efforts is to support Intercept’s ongoing operations.
Financial Highlights
For the fiscal quarter ended June 30, 2020, Sanwire announced significant revenue growth related to the acquisitions of Intercept Music and Art is War Records. Since acquiring Intercept Music in March and Art is War Records in June, Sanwire’s revenue has increased by approximately 300% (http://nnw.fm/j0S0j). Sanwire attributes the increase in revenue to Intercept Music’s customer acquisition and the release of its PLUS plan.
For the third quarter, revenue is expected to continue an upward climb, owing largely to physical distribution plans and a rising number of PLUS subscribers. The company’s acquisition of Art is War Records is also expected to fuel this growth.
Management
Christopher M. Whitcomb is the current CEO of Sanwire Corp. and Intercept Music Inc. He is a CPA in the state of California, holding bachelor’s degrees in accounting, corporate finance and business management with a focus on real estate. A seasoned executive, his business ventures are always strongly focused on the development and financing of companies.
Whitcomb worked alongside Ralph Tashjian at SMC Entertainment Inc. and Digital Music Universe. They are currently working together again following Sanwire’s acquisition of Intercept Music, which was founded by Tashjian.
Save Foods Inc. (NASDAQ: SVFD)
Save Foods Inc. (NASDAQ: SVFD) is an agri-food tech company focused on developing and selling eco-friendly products specifically designed to ensure food safety and extend the shelf life of fresh fruits and vegetables. The company is focused on addressing two of the most significant challenges faced by the industry: (1) food waste and loss, and (2) food safety.
Fungi like mold and yeast, as well as foodborne pathogens, are typically responsible for fresh produce spoilage and foodborne illness. Save Foods’ integrated solutions improve safety, freshness and quality every step of the way, from field to fork. The company’s natural products control human and plant pathogens, allowing growers, packers and food retailers to reduce waste and boost revenues. More food ends up on consumers’ plates, and less ends up in landfills.
Save Foods’ products use all-natural ingredients to protect fresh produce from microbial spoilage and pathogens with zero toxicity. The company’s treatments leave no harmful residues on produce or in the environment and maintain product freshness over time. Fresh produce treated with Save Foods’ products can already be found in supermarket chains across the U.S. and Europe. Those chains have reported that the company’s products are reducing fruit spoilage by 50% on average at the retail level. With no need for additional steps in the treatment process nor special equipment, Save Foods’ products are easy to implement and come in versatile applications suitable for the different stakeholders along the food supply chain.
Initial applications for the company’s offerings include post-harvest treatments in fruit and vegetable packing houses that process citrus, avocados, pears, bell peppers and mangos. By controlling and preventing pathogen contamination and significantly reducing the use of chemicals and their residues, Save Foods’ products not only prolong shelf life; they also ensure safe, natural and healthy food. Save Foods has the first green products that could realistically replace the different chemicals used today in food treatment while controlling waste and food safety.
Products & Technology
- SavePROTECT or PeroStar, a processing aid added to fruit and vegetable wash water and used in post-harvest treatment;
- SF3HS and SF3H, post-harvest treartment solutions to control both plant and foodborne pathogens;
- SpuDefender, for controlling post-harvest potato sprouts; and
- FreshPROTECT, for controlling spoilage microorganisms on post-harvest citrus.
Save Foods’ products are based on a proprietary blend of food acids which have a synergistic effect when combined with certain types of sanitizers and fungicides at low concentrations in a non-organic setting. The combination eliminates fungicide residues or reduces them to levels below the established Maximum Residue Levels (MRLs). The company’s fruit and vegetable wash is odorless and does not irritate human eyes, skin or airways. Save Foods’ blend does not leave any residues of toxicological concern on the treated surface of produce, and all its ingredients are classified by the U.S. Food and Drug Administration (FDA) as Generally Recognized As Safe (GRAS). There are 7 patent families related to Save Foods’ technology.
Applications
The company’s products have been commercially validated on citrus, mangos, avocados, pears, bell peppers, microgreens and various fresh cut vegetables. Save Foods is in the validation process for bananas, apples, figs, berries, lettuce, papayas and more. The company is also validating the efficacy of its products for pre-harvest treatment, starting with citrus trees.
Market Outlook
The world population is expected to grow to almost 10 billion by 2050, boosting current agricultural demand by some 50%. Providing healthy and safe food for the world’s population is one of the biggest challenges of the 21st century.
Globally, around 664 million tons of fresh fruits and vegetables are lost every year from field to fork, wasted by spoilage, and almost one in 10 people globally falls ill every year from eating contaminated food, with an estimated resulting cost around $90 billion.
Disposing of all that wasted food requires additional expense and harms the environment with resulting greenhouse gas emissions. The post-harvest food treatment market was valued at $1.5 billion in 2019 and is expected to grow to $2.3 billion by 2026, achieving a CAGR of 6.5%.
Management Team
David Palach is CEO of Save Foods. He spent over a decade with Intel Israel, where his last position was Manager of Business Development for Israel and Europe. Prior to that, he served as a controller of two of Intel’s largest factories in Israel, where he supervised a budget of over $1 billion. He also served as the CEO of B-Pure Corporation Ltd., a management and maintenance company involved in protecting and improving the environment. During his tenure, he helped turn around several struggling subsidiaries and made them profitable.
Vered Raz Avayo is the company’s CFO. Before joining SaveFoods in 2018, she spent more than 10 years as CFO at LGC, the Leviev Group of Companies. She has operated her own financial and business consultancy and has served as a director for a number of public companies in Israel.
Dan Sztybel is CEO of SaveFoods Ltd., the Israeli subsidiary of Save Foods Inc. He previously led the Life Sciences Advisory at EY Israel and early on recognized the potential of Israel as a center of innovation in the digital health space. He has been an adviser on digital health strategy to large pharmaceutical companies and is a cofounder of MyndYou, a digital health start-up focusing on cognitive impairment. He is also a co-founder of the DigitalHealth.il conference, the largest digital health conference in Israel.
Dr. Neta Matis is Vice President of R&D at Save Foods Ltd the Israeli subsidiary of Save Foods Inc . She holds a Ph.D. in organic chemistry and an MBA from Tel Aviv University. Prior to joining Save Foods in 2019, she held multiple research chemist and product development roles at Verdia Inc. and its parent company, Helsinki-based Stora Enso Oyj.
Nimrod Ben Yehuda is the founder and CTO of Save Foods Ltd. He was previously the CEO/CTO of Swissteril Water Purifications Ltd. He has also been CEO at Nir Ecology Ltd., and was Joint-CEO at NitroJet Ltd.
Dr. Art Dawson is the U.S. Business Manager for SaveFoods Inc. He has been president of The Dawson Company, which focuses on creating sales opportunities for new agricultural technologies, previously Dr. Dawson held senior industry positions like General Manager Worldwide of the Decco , the Post Harvest Division for Elf Atochem. He holds a Ph.D. in Plant Physiology from UC Riverside and is licensed in California as an agricultural Pest Control Advisor.
Sharing Services Global Corporation (SHRG)
Sharing Services Global Corporation (SHRG), formerly Sharing Services Inc., is a diversified company dedicated to maximizing shareholder value, operating through two primary subsidiaries: Elepreneurs Holdings, a direct-selling company, and Elevacity Holdings, a products company. Headquartered in Plano, Texas, SHRG markets and distributes Elevate-branded health and wellness products through an independent sales force of distributors called Elepreneurs.
Proprietary Products
SHRG’s current exclusive Elevate product offerings are marketed under the Elevacity brand, so named to signify the company’s commitment to elevating lives.
The Elevate health and wellness product line consists of nutraceutical products that SHRG refers to as D.O.S.E., which stands for dopamine, oxytocin, serotonin and endorphins – all of which are key hormones proven to promote happiness and well-being.
Elevacity brand products are carefully formulated, chosen and designed to support a single objective: elevate the happiness and well-being of the consumer.
Global Network of Elepreneurs
Elevacity products are shared and sold by a growing international network of home-based entrepreneurs, called Elepreneurs, operated by Elepreneurs Holdings. This SHRG subsidiary provides basic and advanced programs for both new and experienced entrepreneurs who are focusing on their direct-sales careers.
SHRG’s high-performing independent sales force follows the company’s Blue Ocean selling strategy, an approach that encourages individuals to seek new markets, lead, and to “stop competing and start creating.” The Blue Ocean strategy is based on the book, “Blue Ocean Strategy,” written by Professor Renée Mauborgne, who notes that “the lesson here is that the best defense is offense, and the best offense… is to make a blue ocean shift and create your own blue ocean.”
Following this selling strategy, SHRG’s Elepreneurs are taught that, rather than competing directly in a competitive, direct-selling market, they should focus on making competitors irrelevant and succeeding in an uncontested marketplace.
In addition, SHRG’s Elepreneurs use the interactive, video-based VERB sales-marketing platform developed by Verb Technology Company Inc. The app utilizes proprietary interactive video data collection and analysis technology and provides next-generation customer relationship management, lead generation, and video marketing software applications.
Continued Momentum as Industry Leader
These selling strategies have resulted in sharp and consistent revenue gains. In the company’s 10-Q filed with the SEC for the three months ended Oct. 31, 2019, SHRG reported sales of $38.8 million for fiscal Q2 2019, an increase of 116% over sales of $17.9 million reported for the comparable quarter of 2018. Consolidated gross profit jumped by $16.2 million to $27.4 million for the same period compared to Q2 2018.
SHRG’s consolidated operating earnings were $3.9 million in the fiscal quarter ended Oct. 31, 2019, compared to $866,802 for the comparable period the prior year. Consolidated gross margin also grew 70.9% for the three months ended Oct. 31, 2019, compared to 62.2% the prior year.
These numbers are continuing a trend established over the past two years. In fiscal Q1 2019, SHRG achieved revenues of $35.4 million, more than double that of the comparable period in 2018. Even earlier, the company reported sales of $85.9 million for fiscal year ended April 30, 2019. This represents a nine-fold increase, or $77.5 million jump, over the company’s revenues of $8.4 million the prior year.
These numbers bring SHRG’s sales revenues since December 2017 — when the company’s Elevate product line was released — to an impressive cumulative total of $169 million.
Preparing for Success
SHRG is well prepared to continue and accommodate for this growth. The company recently expanded its corporate footprint by moving to a 10,000-square-foot facility in Plano, Texas, that offers ample room to expand as the company grows and flourish. The larger corporate locale provides space for a growing customer service department, product fulfillment, opportunity and training rooms, as well as a video production suite.
In addition, the company has a seasoned, expert leadership team in place, led by John “JT” Thatch. Thatch was appointed president and CEO of SHRG in March 2018, bringing to the company his expertise obtained from successfully starting, owning and operating several businesses in various industries. His experience with corporate growth, acquisitions, financing and negotiation in fast-paced and flexible environments will significantly assist SHRG as the company aims to expand and increase revenues.
Contact
469.304.9400 x 201
Info@SHRGinc.com
http://www.SHRGinc.com
Sigma Additive Solutions (NASDAQ: SASI)
Sigma Additive Solutions (NASDAQ: SASI), doing business as Sigma Additive Solutions, is the only provider of in-process quality-assurance software to the commercial 3D printing metal industry that enables operators of machines making 3D metal parts to offset emerging quality problems, sustain part quality, and avoid rejects. Sigma’s software is the singular solution that enables both real-time, in-process detection of quality control manufacturing irregularities for critical metal parts and then provides the operator the actionable information needed to adjust and mitigate the developing anomaly. Sigma’s software represents a paradigm shift in the quality control process for the manufacture of 3D printed metal components. The nascent 3D metal printing industry is on the verge of radically altering the speed and technical complexity of manufactured parts. Further, it makes possible just-in-time availability of critical components – all at reduced cost, time, waste and weight. 3D printing, heralded as the fourth industrial revolution in manufacturing, will only truly surpass traditional techniques when the additive manufacturing industry moves from “post process” quality control to “in process” quality assurance.
For the industry to move from prototype manufacturing of critical components to economically viable commercial production, the 3D metal printing industry must find ways to dramatically increase production speed and quality yields, and to dramatically decrease the excessive cost of quality control. To achieve these prerequisites and move 3D metal printing into the mainstream, parts must be inspected and certified during the manufacturing process rather than after. Parts in the production process that are developing signs of quality control problems must be identified in real-time and alerts must be issued. The problem, along with the solution, must then be communicated to the machine operator to implement repairs.
Revolutionizing Additive Manufacturing
Sigma, with its PrintRite3D® brand, has established a new benchmark in the development and commercialization of real-time computer aided inspection (“CAI”) solutions. Sigma resolves the major roadblocks and costly quality control challenges that impede the 3D manufacture of precision metal parts. The company’s breakthrough computer-aided software product revolutionizes commercial additive manufacturing, enabling non-destructive quality assurance during production, uniquely allowing errors to be corrected in real-time.
Sigma was founded in 2010 by a team of Los Alamos National Labs scientists and engineers to develop and commercially license advanced metallurgical products for the military ordinance, dental implants, and then for additive manufacturing (3D printing). After assessing 3D metal printing technology and the costly, inconsistent quality control issues, Sigma concluded that the enormous potential of 3D metal printing could only scale up if in-process quality-assurance tools were developed to observe, manage and control the manufacturing complexities in such a manner that reliability and repeatability of very high precision quality metal parts could be achieved in the process. Sigma’s patented and third-party validated software has achieved these objectives and now delivers the critical elements needed to unleash the promise of 3D metal printing.
Sigma’s products and services are engineered, manufactured and qualified for use in the highly demanding and hyper precise production environments of the aerospace, defense, transportation, oil and gas, biomedical and other precision-dependent industries.
The Challenge
Additive metal manufacturing combines multiple processes and parts into one single 3D printed part. Due to variances in the additive manufacturing process, parts of consistent quality currently can’t be reliably produced in either large or small quantities without substantial postproduction inspection and rejection costs. Parts are inspected after production using CT scans and other means, so the manufacturer doesn’t know until the very end which of the finished parts meet design specifications. This means lost time, lost profits and inability to economically scale up production.
Innovative Approach
Sigma solves this problem with its patented, in-process quality control technology that informs operators and engineers how to improve both the manufacturing process and quality by capturing meaningful data about inconsistencies in real-time. Sigma is also partnering with OEMs, working toward the visionary introduction of revolutionary closed-loop control that will bypass the machine operator and automatically make in process corrections by reducing machine variations.
Sigma’s next generation technology gives manufacturers the ability to make fast, virtual real-time adjustments so that each finished part is uniform and within critical specifications, thereby improving production quality, decreasing end-users’ risks and waste, and increasing profits and speed to market. Sigma’s PrintRite3D® IPQA Software monitors and assesses the quality of each production part in the 3D additive manufacturing process – layer by layer, and in real-time. This has never been available until now.
Sigma maintains a strong intellectual property portfolio consisting of trade secrets, process know-how and 34 patents either granted, pending or awaiting pre-publication around the globe. These patents encompass the fundamental technologies underlying Sigma’s melt pool process control, data analytics, anomaly detection, signature identification, and future “closed-loop control” of 3D metal printing.
Market Opportunity
Providing advanced quality assurance software to the commercial 3D printing industry is currently a $1.4 billion addressable market expected to grow to $3.9 billion by 2023. Integrating Sigma’s groundbreaking software helps arm the industry with a necessary catalyst to help enable and optimize the fourth industrial revolution in manufacturing.
Sigma’s global client base includes 23 installations across 19 different users. Tier-1 OEM enterprises and end-users such as Siemens, Honeywell, Pratt & Whitney and others are currently evaluating PrintRite3D® for production lines.
Management Team
John Rice, CEO and chairman of the board of directors, has extensive experience as a CEO, lead negotiator, turnaround expert, business financier and crisis management executive/consultant. Prior to becoming chair and CEO of Sigma, he was the CEO of a successful turn-around of a Coca-Cola Bottling Company. Rice has led a variety of companies in diverse business sectors and worked on a host of products and technologies including design and manufacture of high-end jet engine test equipment for the U.S. Airforce, chaff dispensers for F16s, software for modeling naval exercises, software for controlling warehouse distribution systems, medical radioisotopes, cancer detection, and cybersecurity. He is an honor’s graduate of Harvard College.
Darren Beckett, CTO, has over 20 years of experience in the semiconductor industry, including Intel Corporation, where he held various technical and managerial positions. His expertise in process engineering for advanced manufacturing technology includes statistical process control for fabrication of semiconductor devices.
CFO Frank D. Orzechowski also serves as treasurer, principal accounting officer, principal financial officer and corporate secretary. He has more than 30 years of distinguished financial and operational experience. Orzechowski began his career at Coopers & Lybrand in 1982, received his CPA certification in 1984, and received his Bachelor of Science in Business Administration with a major in accounting from Georgetown University in 1982.
Ronald Fisher, vice president of business development, is leading the commercialization of PrintRite3D® 5.0. Fisher is a mechanical engineer with hands-on experience in quality, manufacturing and product development. He has distinguished himself as a lead sales and marketing officer as well as a chief operating officer most recently before joining Sigma in technology startup that grew from market entry to successful exit by merger-acquisition.
Silo Pharma Inc. (OTCQB: SILO)
Silo Pharma Inc. (OTCQB: SILO), a developmental stage biopharmaceutical company, is focused on merging traditional therapeutics with psychedelic research for people suffering from indications such as post-traumatic stress disorder (PTSD), fibromyalgia, Alzheimer’s disease, Parkinson’s disease, and other rare neurological disorders. Silo’s mission is to identify assets to license and fund research that the company believes will be transformative to the wellbeing of patients and the health care industry.
Silo is committed to developing innovative solutions to address a variety of underserved conditions. Combining Silo’s resources with world-class medical research partners, the company looks to make significant advances in the medical and psychedelic space.
Silo works to identify and partner with leading medical universities, providing the needed financial resources to develop safe therapeutic treatments while moving cutting-edge research through the clinical stage and into commercialization. The company is well-capitalized with access to additional funds as opportunities present themselves.
Silo recently engaged Donohoe Advisory Associates LLC for consulting and advisory services in connection with the potential uplisting of Silo’s common shares to the Nasdaq Stock Market.
Research
Silo has entered into research agreements and partnerships with multiple leading medical universities.
The company is involved in a sponsored study with Maastricht University utilizing repeated low doses of ketamine and psilocybin to examine the effects on cognitive and emotional dysfunctions in Parkinson’s disease and to understand its mechanism of action. The investigator in the Netherlands is acquiring the substances for the study and will then finalize the documentation to submit to the ethics committee.
Additionally, in June 2021, Silo announced its entry into a scientific research agreement with the University of California San Francisco (UCSF). The agreement will leverage four other clinical trials being planned by the university to determine the effects of psilocybin on inflammation. The study will take place at The Translational Psychedelic Research (TrPR) Program at UCSF.
Silo also recently extended its exclusive option agreement with the University of Maryland, Baltimore (UMB) to explore a novel invention generally known as joint-homing peptides. These peptides are being developed for use in the investigation and treatment of arthritogenic processes and can be used for enhanced targeting of therapeutic agents.
This agreement includes the study of two separate peptides. The first is an option and study for the treatment of arthritis. The second is a patented licensed peptide for the central nervous system, with an initial study for MS autoimmune diseases, in addition to rheumatoid arthritis. Animal studies are underway for both initial indications relating to the UMB agreement, with the potential for studies evaluating additional indications in the future.
Finally, Silo signed an agreement with Columbia University granting it an option to license certain assets currently under development, including an Alzheimer’s disease formulation targeting NDMARs and 5-HT4Rs, as well as a prophylactic treatment for stress-induced disorders and PTSD. Both candidates are currently being tested in mice and have already provided early data.
In addition to its university partnerships, Silo entered a joint venture agreement with Zylo Therapeutics Inc. (“ZTI”) focused on the development of ketamine and psilocybin using ZTI’s Z-Pod™ technology for the transdermal time released delivery of therapeutics. In November 2021, the company announced ZTI’s reception of its first ketamine shipment and initiation of loading ketamine into its Z-Pod technology. In a news release, Eric Weisblum, CEO of Silo, called the development an “important milestone” that will help the company “study the benefits of slow-release transdermal release of Ketamine.”
Market Overview
According to Coherent Market Insights, the fibromyalgia treatment market was valued at $2.78 billion in 2018 and has a projected CAGR of 3.3% over the forecast period 2018 to 2026. Fibromyalgia is a condition that causes pain all over the body, sleep problems, fatigue, and emotional and mental distress.
The global PTSD therapeutics market is expected to reach $10.68 billion by 2026 with a CAGR of 4.5% during the forecast period from 2018 to 2026, according to a report by Credence Research. Growing prevalence of PTSD is the chief factor driving the global treatment market. Increases in events such as wars, combat, and interpersonal violence has been a major contributing factor. Other factors like growing emphasis on rehabilitation initiatives by governments for treating their war veterans has also been facilitating the increase in demand for PTSD therapeutics.
Fortune Business Insights reports the global Parkinson’s disease treatment market is predicted to grow to $8.38 billion by 2026, with a CAGR of 8.1% during the forecast period. Parkinson’s is a neurodegenerative disease of the central nervous system which primarily affects the brain, causing uncontrollable shaking and tremors, difficulties in balance and restricted body movement making it difficult for the person to function or perform a daily routine.
Management Team
Eric Weisblum is CEO and founder of Silo Pharma. He has over 25 years of Wall Street experience, most recently in the biotechnology sector. He has served on the board of Aikido Pharma and was the president of Sableridge Capital. He has a proven track record in licensing therapeutic assets and assisting in their development. He brings to the company nearly 20 years of expertise in structuring and trading financial instruments. He holds a bachelor’s degree from the University of Hartford’s Barney School of Business.
Dr. Kevin Muñoz was appointed to the Silo board of directors in October 2020. He teaches biomedical sciences and medical intervention for the Passaic County Technical Institute. He previously served as Director of Operations at Physical Medicine and Rehabilitation. He began his career with Harlem Health Promotion Center in New York City as a research assistant. He earned a bachelor’s degree from the University of Michigan and a Doctor of Medicine from Xavier University School of Medicine.
Josh Woolley, M.D., Ph.D., is a Scientific Advisor for Silo. He is an associate professor in the Department of Psychiatry and Behavioral Sciences at the University of California, San Francisco. He is also a psychiatrist on staff at the San Francisco Veterans Affairs Medical Center. He is the director and founder of the Bonding and Attunement in Neuropsychiatric Disorders Laboratory. He received both his M.D. and his Ph.D. in Neuroscience from UCSF, where he completed his psychiatry residency training.
Charles Nemeroff, M.D., Ph.D., is a Scientific Advisor for Silo Pharma. He directs the Institute for Early Life Adversity Research within the Department of Psychiatry and Behavioral Sciences as part of the Mulva Clinic for the Neurosciences. He was chair of the Department of Psychiatry and Behavioral Sciences and clinical director of the Center on Aging at the University of Miami Miller School of Medicine. He received his M.D. and Ph.D. in neurobiology from the University of North Carolina School of Medicine.
Siyata Mobile Inc. (TSXV: SIM) (NASDAQ: SYTA)
Siyata Mobile Inc. (TSXV: SIM) (NASDAQ: SYTA) is a leading global developer and provider of Push-to-Talk Over Cellular (“PTT/PoC”) systems for enterprise customers. The company specializes in connected vehicle products for professional fleets and markets its products under the Uniden® Cellular brand.
Since its inception in 2012, Siyata has amassed a customer base that includes cellular operators, commercial vehicle technology distributors, and fleets of all sizes in Canada, the U.S., Europe, Australia and the Middle East.
Recognized by the Toronto Venture Stock Exchange in 2018 as a Venture Top 50 Company, Siyata aims to deliver the highest quality and most technologically advanced mobile communication devices for global corporate workforces, fleets, homes and buildings.
The company has long been an industry pioneer, delivering the world’s first 3G connected vehicle device as well as the world’s first 4G/LTE vehicle-mounted smartphone for First Responders and commercial fleets and vehicles, thereby creating a new category in the cellular device market with a dedicated smartphone tailor-made for the commercial vehicle market.
Siyata is headquartered in Montréal, Québec, Canada.
Product Portfolio
Siyata’s suite of technology includes numerous PTT and legacy devices, as well as cellular boosters designed to improve cellular signals in corporate warehouses, government embassies, retirement home campuses, banks and manufacturing plants.
The company’s flagship product, the Uniden UV350, is the world’s first vehicle-mounted 4G/LTE smartphone with crystal clear quality, carrier grade PTT, voice, text, video and data applications built into a single device. Specifically designed for First Responder and commercial fleet vehicles, the UV350 runs on cellular LTE networks that provide nationwide and global coverage, replacing traditional single purpose two-way radios that require a monthly fee and limited network coverage.
The Uniden UV350 is currently available through Bell Mobility, Canada’s largest LTE network and PTT community, as well as AT&T in the U.S. Further expanding its availability, Siyata is completing network approval with another U.S. Tier 1 operator to launch the UV350 in Q3 2019.
Management Team
CEO and Chairman Marc Seelenfreund is the founder of Siyata. He is also the founder of Siyata’s parent company, Accel Telecom, an Israel-based company that specializes in importing and distributing innovative cellular and IP devices to fixed line operators and mobile providers within Israel. Prior to establishing Accel, Seelenfreund was a vice president at Sunrise Corporation in New York where he focused on financing publicly traded technology companies. Seelenfreund has a law degree from Bar Ilan University, is a board member at Israel’s leading private university, and has served as an officer in the Israel Defense Forces.
Glenn Kennedy, vice president of sales, has over 25 years of sales experience in the telecommunications industry. Prior to joining Siyata in 2016, Kennedy managed sales nationally for Motorola Canada, HTC Communications Canada, and Sonim Technologies. He holds a bachelor’s degree in honors business administration from the Richard Ivey School of Business at the University of Western Ontario.
CFO Gerald Bernstein, a professional chartered accountant, has spent 20 years focusing on private equity financing and tax efficient corporate structuring in multi-jurisdictional arenas. He holds a bachelor’s degree of commerce as well as a graduate diploma in public accountancy from McGill University. Bernstein has been a member of the Canadian Institute of Chartered Accountants since 1987.
Gidi Bracha, vice president of technology, has served in this position since 2011 and spearheaded the development of both the Truckfone, Voyager and UV350. Bracha served in various key positions at Cellcom, Israel’s leading cellular provider, including head of car mobility products and director of type approvals. Bracha served as an engineer technician in the Anti-Aircraft division of the Air Force in the Israel Defense Forces and holds a bachelor’s degree in engineering and business management from the University of Derby.

Simply Sonoma Inc. produces healthy CBD medicinals and beauty products for the environmentally conscious consumer. The company strives to create the best sustainably grown natural medicinal alternative products and is committed to minimizing its carbon footprint by powering operations off-the-grid using solar energy. Simply Sonoma is creating unique medicinal hemp strains that are alternatives and supplements to traditional, chemically manufactured therapies. The company believes in all-natural, organically sun-grown, plant-based medicinals, and it provides consumers with science-based education on CBD for disease and lifestyle needs.
Simply Sonoma is focused on being a leader in the industry for plant-based medicinal health and beauty products and partnering with like-minded organizations. The company strives to develop broad-spectrum CBD products for therapeutic applications from a scientific perspective. Its products come from the farm rather than from a lab, with the goal of achieving fewer side effects and more efficacy for patients. The company believes in published, science-based trials and research with regard to its CBD creations.
Simply Sonoma is a different kind of natural company. From seed to sale, it owns or contracts the organic grow, extraction and product formulation operations. The company has been developing products since 2017 based on scientific research and data and has several ready to launch. Its nationally available organic CBD products employ the company’s own proprietary formulations.
For example, the company’s nonalcoholic CBD Pinot Noir beverage uses grapes specially grown for Simply Sonoma and is infused with CBD from the company’s farm. The company expects to generate revenue through national sales of its CBD products via e-commerce, as well as through a variety of chain stores, pharmacies and small businesses throughout the U.S.
Products
Simply Sonoma has three tiers of products:
- Organic CBD formulations for consumer medicinal applications.
- Organic whole plant extracts of CBD and cannabinoids – providing the whole plant synergistic effect and giving a dose response for a variety of diseases.
- Organic extracts paired with traditional over-the-counter functionality, delivering all the benefits of traditional OTC products but the bulk product is organic and plant derived for a more natural healthy approach, minimizing synthetic chemical components and adverse effects.
An example of tier three would be the company’s sleep aid. Current over-the-counter and prescription sleep aids like benzodiazepines, antihistamines and sleeping pills disrupt normal sleep brain patterns including REM, resulting in abnormal sleep. CBD and cannabinoids have efficacy as sleep aids and do not disrupt the normal sleep cycle.
Depending on the application, the company’s products can be delivered via:
- Teas
- Pills
- Inhalers
- Skin patches
- Gummies
- Tablets
- Sublingual sprays
- Tinctures
- Topicals
- Juices
Simply Sonoma has partnered with Sonoma Biologics, a premium hemp cultivator that has completed considerable research on the scientific nature of hemp and cannabis, to grow proprietary medicinal strains specifically for the company. Additionally, Simply Sonoma is working with Organic Vineyards on the company’s antioxidant, alcohol free, CBD wine product, as well as its low carb, low sugar Pinot Noir CBD sparkling product. All partner companies are environmentally conscious, solar first and organic-equivalent. Simply Sonoma’s CBD products will contain less than 0.3% of THC.
Market Outlook
Simply Sonoma competes with numerous nondescript CBD companies in the market today. The company feels its major competitive advantage is its scientific staff and product formulation expertise. Simply Sonoma products are focused on four specific health, beauty and lifestyle markets, including sleep aids, joint pain, probiotics and skin health. The Market Data Forecast valued the global sleep aid market at an estimated $175 billion in 2020. The joint pain and anti-inflammatory market is forecast to be worth approximately $41 billion in 2026 by Persistence Market Research. The market for gut health and probiotic products is expected to hit $65 billion by 2023, according to Global Market Insights. Allied Market Research valued the global beauty and skin product market at $380 billion in 2019.
Management Team
Simply Sonoma’s dynamic team has a unique combination of experience that positions them well with the company’s wellness and lifestyle products in the CBD space.
Margaret C. Caracciolo is the CEO of Simply Sonoma. She has spent most of her career in biotechnology, in the areas of clinical research and financials. She has worked for notable biotech companies including Heartport, an innovator of heart therapies. She spent time at Aviron, supporting the development of its innovative nasal flu inoculation product, and Genitope, which created personal gene therapies. She has spent the last 10 years co-managing her family’s farm and vineyard and creating wines and other products from the farm’s organic gardens.
Angela Miller is Vice President of Operations at Simply Sonoma. She has extensive experience in cross line-of-business, global project management, and analysis from inception to post-go-live. She spent more than 20 years working at Oracle Corp. and Sun Microsystems Inc., where she obtained expertise in global products, team building, troubleshooting, and customer relations. She also worked seven years with Schwinn Cycling & Fitness, doing everything in the Fitness Division from project management to marketing and public relations.
Splash Beverage Group Inc. (NYSE American: SBEV)
Splash Beverage Group Inc. (NYSE American: SBEV) is a portfolio company of successful beverage brands with the objective of driving value through superior production, supply chain efficiency and global distribution capabilities.
Specializing in manufacturing, distributing, sales & marketing of various beverages across multiple channels, the company operates in both the alcoholic and non-alcoholic beverage segments, allowing it to leverage efficiencies and dilute risk. The company’s business strategy is to quickly develop and/or accelerate pre-existing brands to exit for cash events. Led by a highly successful management team, the company only works with brands it perceives to have highly visible preexisting brand awareness or pure category innovation, thus breaking through the clutter. Splash seeks out brands offering products that:
- Deliver natural quality, health benefits, freshness and refreshment within their beverages;
- Are on trend with consumers;
- Have a high level of brand awareness;
- Maintain highest performance standards and focus on execution;
- Help distributors and retail partners achieve and exceed all goals; and
- Offer unapologetic support for members of the U.S. armed forces, first responders and health care professionals.
Splash was founded in 2013 and is located in Fort Lauderdale, Florida.
Splash Portfolio
The current Splash portfolio includes four unique beverage brands. Each of these brands offers one or more of the qualities that the company specifically seeks in an acquisition.
- TapouT Performance is a natural isotonic hydration & recovery sport drink featuring a 3-in-1 advanced formula. TapouT Performance restores what the body loses through physical exertion, delivering hydration and cellular recovery. Perfectly balanced with key vitamins & minerals and all five necessary electrolytes, TapouT increases nutrient absorption, allowing the body to recover quickly and more efficiently. TapouT is the official training partner of the WWE (NYSE: WWE).
- Salt Naturally Flavored Tequila is a 100% blanco agave 80 proof tequila that offers a clean and delicate taste. Salt is grown, distilled and bottled in the Jalisco region of Mexico. Every bottle of Salt Tequila is the result of hard work, determination and numerous blends. The brand offers a line of tequila flavors for enhanced refreshment, including berry, citrus and salted chocolate.
- Copa Di Vino is the leading producer of premium “wine by the glass” in the U.S. Produced in the Columbia Valley, Copa di Vino is readily available on the go without the requirement of a bottle, corkscrew or glass. Open, drink and enjoy.
- Pulpoloco Sangria is a premium crafted sangria imported from Spain. Its flavor is light-bodied, fruity and refreshing, offering the best blend of Spanish ingredients. The product is filled and packed in a unique eco-friendly biodegradable catocan, allowing Pulpoloco to extend the shelf life of the sangria without the use of preservatives.
Market Outlook
The global beverage industry was valued at $1.5 trillion in 2018 and is projected to grow at a CAGR of 3.1%, reaching a market size of $1.9 trillion by 2024 (https://nnw.fm/w1Cx9). The push for non-alcoholic beverages that are healthier and contain zero sugar is expected to be a driving force in the forecast period and beyond.
With a seasoned management team and sufficient capital to fuel sustained growth, Splash is uniquely positioned to capitalize on this market growth. The company is currently preparing a secondary offering and has engaged Kingswood Capital Markets as lead underwriter in order to uplist to the Nasdaq or NYSE in the near future.
Management Team
Robert Nistico is the Chairman and CEO of Splash Beverage Group. He has 28 years of experience in the beverage industry and was the fifth employee and SVP/General Manager of Red Bull North America. In this role, he led the start-up from zero sales to $1.65 billion in annual sales. Mr. Nistico was a founder and President of Marley Beverages and was responsible for framing the company’s long-term vision. Mr. Nistico held executive positions at DIAGEO, Republic National Distributing Company and the Gallo Wine Company resulting in decades of successful experience in the ‘Three Tier Beverage System’. In the spirit of his true entrepreneurial nature, he is a motivated, results-driven, creative and passionate leader.
William Meissner is the company’s President and CMO. He boasts over 20 years of success in growing consumer brand companies with large and medium-sized entrepreneurial organizations, both locally and internationally. His résumé includes multiple CEO roles, leading efforts to revamp both healthy and distressed companies. Before joining Splash, Mr. Meissner was the President and CEO of Sweet Leaf and Tradewinds Tea. He has held multiple positions with leading companies in the beverage sector, including Sparkling Ice, Jones Soda, SoBe Beverages, Fuze & NOS (Coca-Cola) and many others.
Sanjeev Javia is the Vice President of Product Development for Splash. He is the founder and President of Javia Wellness Group, a firm focusing on the innovation, research, formulation and design of healthy exercise and wellness initiatives. Mr. Javia is a sports nutrition expert, allowing him the advantage of developing innovative functional beverages that include health benefits for consumers. Since 2000, he has advised and written nutritional plans for hundreds of the world’s most famous athletes, including Tom Brady, Kurt Warner, Curt Schilling and more.
Dean Huge is the company’s Chief Financial Officer. He brings 35 years of public and private sector accounting and finance experience to the Splash Beverage team. Mr. Huge has led four public offerings as CFO and guided the growth efforts of numerous companies, including Catalyst Energy Corp., which was named Inc. Magazine’s ‘Fastest Growing Company’ within 36 months of his joining. His expertise spans financial services, manufacturing, distribution and SAAS-type programs.
Aida Aragon is the company’s Senior Vice President of National Accounts. She is a sales, marketing and brand management executive with years of experience working in the sports supplement and beverage industry. In her previous positions, Ms. Aragon was vital in leading successful store rollouts for brands including Muscle Milk. Her passion for brand development comes as second nature, but her true passion has always been focused on increasing sales for brands in the sports nutrition industry.
SPYR Inc. (OTCQB: SPYR)
SPYR Inc. (OTCQB: SPYR), dba SPYR Technologies, is a technology company which, through its Applied MagiX Inc. subsidiary, develops and resells Apple®-ecosystem-compatible products with an emphasis on the growing, multibillion-dollar Internet of Things (IoT) Smart Home and Connected Car markets.
SPYR continues to identify and target acquisitions with an aim of growing its footprint in the industry and expanding the products it offers consumers, including companies developing artificial intelligence and smart-technology products. In 2020, SPYR acquired Applied MagiX Inc., a registered Apple developer and reseller of Apple ecosystem compatible products with an emphasis on the smart home market, as a wholly owned subsidiary. Applied MagiX operates in the IoT market and, more specifically, the segment of the market related to the development, manufacture and sale of devices and accessories specifically built on Apple’s HomeKit® framework. These products work within the Apple HomeKit ecosystem and are exclusive to the Apple market and its consumers.
Initially, while working to develop, manufacture and sell its own line of branded products, Applied MagiX will be sourcing HomeKit products and accessories from worldwide manufacturers, vetting and selecting best-of-breed products, selling them directly to consumers and supporting them. The company focuses on Apple consumers – a target market with higher disposable income and a demonstrated willingness to pay a premium for quality products. On average, Apple product users spend roughly twice as much on technology as other smartphone users. Those who purchase smart home products spend more than $3,000 on average.
By creating smart hardware and software solutions exclusively for Apple consumers, SPYR addresses a problem faced by that market – having few “smart” devices that integrate with Apple’s HomeKit, despite being the most affluent and loyal consumers of tech products.
Products
The company’s Applied MagiX subsidiary offers multiple product lines to its target markets. First, the subsidiary is a reseller of third-party manufactured Apple HomeKit and Apple CarPlay compatible products. HomeKit comes pre-installed on every new iPhone, while the CarPlay platform is licensed by all major auto manufacturers. Applied MagiX identifies white label products, applies the company’s branding, improves the software and sells these improved products to consumers. Finally, Applied MagiX is developing its own proprietary line of smart home and connected car products, including Apple-compatible home cameras, sensors and alarms, as well as additional Apple-compatible smart car products in the iOS ecosystem.
Among the subsidiary’s products sold to consumers are:
- The MagixDrive Wireless CarPlay adapter, which allows users to access CarPlay wirelessly using their iPhones
- The HomeKit Secure Video Camera with iCloud Storage
- The Multipurpose Sensor with Alarm
- The Environment and Motion Sensor
- The Window and Door Contact Sensor
Market Outlook
According to Statista, the global smart home market is expected to generate revenue of more than $104 billion in 2021. The market is forecast to hit more than $187 billion in revenue by 2025, recording a CAGR of 15.75 percent.
The number of active households in the worldwide smart home market is expected to reach nearly 500 million by 2025. Household penetration is just over 12 percent in 2021 and is projected to nearly double by 2025 to more than 22 percent.
Allied Market Research valued the global connected car market at more than $63 billion in 2019 and projected a CAGR of 17.1 percent, which would push revenue to more than $225 billion by 2027. Allied identified rising consumer demand for connectivity solutions, surging need for constant connectivity, increasing dependency on technology and an upsurge in tech-savvy population as key factors driving the projected growth of the connected car market.
Management Team
James R. Thompson is the CEO, President and General Counsel of SPYR. Over the past 28 years, Mr. Thompson has deftly managed a colorful spectrum of legal clients and situations. In the process, he has helped many companies – both large and small – thrive. Now he welcomes the challenge to take the company and his career in an entirely new direction. A native of Philadelphia, he holds a J.D. from Rutgers University and a Bachelor of Science from the University of Denver.
Jennifer Duettra is the Executive Vice President of SPYR. She brings a great deal of knowledge in mobile gaming and pop culture to the company. She is an attorney and was thrilled by the prospect to combine her law experience with a chance to be creative. She is a native of Colorado and received her Bachelor of Arts in Political Science and Speech Communication from Colorado State University. She holds a J.D. from Harvard University.
Trang Nguyen is the CFO of SPYR. From 2019 to 2020, she served as the Financial Reporting Manager for Del Taco, where she was responsible for the preparation and filing of periodic financial reports with the U.S. Securities and Exchange Commission. From 2016 through 2019, Ms. Nguyen was Accounting Manager for Pinnacle Tax Accounting in Los Angeles, California. She was a part of Ernst & Young’s audit team in Los Angeles from 2006 to 2008, leading engagements on interim and year-end ad SOX 404 auditing procedures for major enterprise accounts. Ms. Nguyen holds a Bachelor of Art, Business Economics (Minor in Accounting) from the University of California, Los Angeles. She is a certified public accountant with an inactive license.
Dr. Harald Zink is the CEO, Founder and Chief Product Architect of SPYR subsidiary Applied MagiX. Prior to founding Applied MagiX, he was Director of Technologies and later Vice President of Technologies at Sarkissian Productions in Los Angeles. He also served as Director of Technologies at SMZ Technologies and, for more than 17 years, as Macintosh Technology Consultant to The Walt Disney Studios in Burbank, California. He speaks five languages and holds degrees from the University of California, Riverside.
Kelly Clark is the COO of Applied MagiX. Before joining the subsidiary, he worked as Vice President of Sales Operations at TruClear Global. Prior to that, Mr. Clark was Senior Director of Program Management at Pacific Group Ventures and Operations Manager at Barco. He has also held operations management positions at Deluxe Digital Studios and Sony Pictures Entertainment. Mr. Clark holds a bachelor’s degree in international business from the University of Southern California.
SRAX Inc. (NASDAQ: SRAX)
SRAX Inc. (NASDAQ: SRAX) is a digital marketing and consumer data management technology company. SRAX’s technology unlocks data to reveal brands’ core consumers and their characteristics across marketing channels.
Through its BIGtoken platform, SRAX has developed a consumer-managed data marketplace where people can own and earn from their data, thereby providing everyone in the internet ecosystem choice, transparency and compensation.
SRAX’s tools deliver a digital competitive advantage for brands in the CPG, automotive, investor relations, luxury and lifestyle verticals by integrating all aspects of the advertising experience, including verified consumer participation, into one platform.
SRAX Verticals
- SRAX Core: SRAX Core is a custom digital media management platform that enables brands and agencies to surpass the challenges of omnichannel marketing campaigns. It offers one comprehensive dashboard to manage digital media campaigns, inventory and reporting.
- SRAX Social: SRAX Social is a free social media management tool that makes it easy for brands, agencies and individuals to grow their digital presence. It offers free and unlimited users, Facebook auto boosting, and a custom analytics dashboard. Its managed services team can also build and execute marketing plans for your unique specific needs.
- SRAX IR: SRAX IR unlocks stock buyers’ behaviors and trends for issuers of publicly traded companies. The platform provides insights on shareholders and market makers, investor relations management, shareholder outreach tools and data-driven marketing.
- SRAX Auto: SRAX Auto unlocks auto intenders’ data to create measurable connected experiences on the road to purchase. It offers proprietary auto intender profiles, multi touchpoint communication and custom location-based ads.
- SRAX Shopper: SRAX Shopper delivers a cross channel, premium digital experience at scale to high value shopper audiences. It offers proprietary shopper profiles, cost per click pricing, and custom text and add to cart ad units.
- SRAX Lux: Launched in June 2019, the SRAX Lux platform targets and reaches luxury consumers at luxury retail stores, high-end art, music, film, fashion and sports events, across all consumer devices.
BIGtoken
BIGtoken, available for download on the App Store and Google Play, revolutionizes data collection. BIGtoken is a platform that creates a secure and transparent environment for consumers to own and earn from their data. To date, there are 15.9 million BIGtoken registered users worldwide.
The optimization and monetization of data is a multibillion-dollar business. Worldwide spending on big data and business analytics solutions reached $166 billion in 2018 and is projected to surge to $260 billion by 2022. BIGtoken’s consumer vision is committed to delivering choice, transparency and compensation to the individual.
Through BIGtoken, consumers earn rewards when they opt into sharing their data and when that data is purchased. Consumers decide what data is shared, who can buy it and how it’s used, and advertisers reach real, responsive audiences. The benefit of this is two-fold: consumers know how their data is used and advertisers gain verified consumer data for targeting.
Users of the BIGtoken app can officially be paid in cash or gift cards in exchange for giving brands access to their anonymized data, answering questions, checking into locations, recruiting new members, and more. Users can deposit their earnings directly into PayPal accounts or be paid through gift cards from favorite retailers such as Walmart.
SRAX has also partnered with several high-profile, nonprofit associations to provide BIGtoken users the ability to donate their earnings. Partnerships include the American Heart Association, dedicated to fighting heart disease and stroke; HealthCorps, which helps high school students make better choices about health and physical fitness; and the ALS Association, which recently launched its Challenge Me campaign.
International Expansion
BIGtoken is formally launching into several international markets and partnering to foster local support. SRAX recently signed a joint venture with the Yash Birla Group to launch BIGtoken in India. Based in Mumbai, the Yash Birla Group, one of India’s largest conglomerates, has diversified interests in consumer and industrial products.
The partnership will bring BIGtoken’s platform to India, which has a digital population of 627 million. The India digital advertising market is $3.6 billion and is set to grow at a compound annual growth rate of 32%, making it one of the largest growing digital ad markets in the world.
SRAX Mexico is led by Moe Avitia, who has more than 18 years of experience in business development and building high-tech teams. SRAX Mexico includes a team of 90 employees, including 70 engineers.
BIGtoken Europe is currently evaluating data centers in individual countries for privacy laws.
Leadership
Christopher Miglino is CEO and founder of SRAX. He has spent the past 20 years working in the digital advertising space and has successfully launched and sold two internet companies. Both of these companies were sold to publicly traded companies on the NASDAQ. He has a detailed understanding of how technology interacts with brands.
Kristoffer Nelson is COO of SRAX and a founding member of BIGtoken. With over 15 years of technology and creative business experience, Nelson has been a guest speaker for Loyola Marymount University among other academic institutions, the National Association of Broadcasters, the IAB and numerous other professional and media organizations.
Standard Lithium Ltd. (NYSE American: SLI)
Standard Lithium Ltd. (NYSE American: SLI) is focused on unlocking the value of existing large-scale U.S.-based lithium brine resources that can quickly be brought into production. The Company believes new lithium production can rapidly be brought on stream by minimizing project risks at selection stage; resource, political & geographic, and regulatory & permitting; and by leveraging advances in lithium extraction technologies and processes.
The Company’s flagship project is in southern Arkansas. The more than 180,000-acre “Smackover Project” is in the most prolific and productive brine processing region in North America. Agreements with large commercial brine operators in the region will allow Standard Lithium to utilize the extensive existing infrastructure, including brine supply and disposal pipelines, water, power and a trained workforce to fast-track project development timelines.
“Arkansas produces about 9.4 billion gallons of brine per year, according to 2010-2016 average statistics reported by the Arkansas Oil & Gas Commission.”
Standard Lithium signed a binding MoU with global specialty chemicals company LANXESS Corporation and its U.S. affiliate Great Lakes Chemical Corporation with the purpose of demonstrating the commercial viability of extraction of lithium from brine (“tail brine”) that is produced as part of LANXESS’ bromine extraction business at its three Southern Arkansas facilities.
LANXESS’ land operations in Southern Arkansas encompass more than 150,000 acres, 10,000 brine leases and surface agreements and 250 miles of pipelines. LANXESS extracts the brine from its wells located throughout the area, and the brine is transported to the three Arkansas plants through a network of pipelines. The three bromine extraction plants currently employ approximately 500 people and process and reinject several hundred thousand barrels of brine per day.
Standard Lithium has developed a breakthrough rapid lithium extraction process that reduces the recovery time of extracting lithium from brine to as little as several hours vs. the current industry method that takes years. The process is also much more environmentally friendly with a significantly smaller footprint than the conventional processes. The company has a signed agreement to locate a demonstration scale lithium extraction plant inside one of LANXESS’ chemical plants in Southern Arkansas.
The Company has also signed an option agreement with NYSE-listed Tetra Technologies for the lithium rights for exploration, extraction, and possible commercial development on approximately 30,000 acres of brine leases in Southern Arkansas. The largest available land package.
Recent laboratory results of four brine samples recovered from two existing wells in Standard Lithium’s project area showed lithium concentrations ranging between 347-461 mg/L lithium, with an average of 450 mg/L lithium in one of the wells and 350 mg/L in the other. Geological modeling of the project area is complete, and a maiden resource report is on the horizon.
Market Opportunity
World demand for lithium continues to surge. The global lithium compounds market is projected to reach U.S. $5.87 billion by 2020 at a compound annual growth rate of 13.22% between 2015 and 2020. Lithium-ion batteries are the fastest growing segment of the market.
Leadership
Standard Lithium’s commitment to being a premier, innovation-driven company focused on developing and commercializing new modern processes for lithium extraction is bolstered by the leading experts that comprise the company’s Scientific Advisory Council. Each member was selected because of their experience and expertise in areas that are central to and/or complement Standard Lithium’s current development plans. Standard Lithium recently welcomed to the Council world-renowned chemist Dr. Barry Sharpless, the recipient of the 2001 Nobel Prize in Chemistry for his work on chirally catalyzed oxidation reactions.
Standard Lithium is led by a team of professionals with proven strong technical and project development skills. CEO Robert Mintak has a global network of industry contacts and is a pioneer in the rapidly evolving lithium space. COO and President Dr. Andy Robinson is an experienced geoscientist with 20+ years of experience and a PhD in Geochemistry from the University of Bristol, UK. Dr. Robinson has worked on a wide range of projects in the resource, power and energy sectors in Europe, Africa, and North and South America.
The company recently appointed Robert Cross as non-executive chairman. Cross is an engineer with 25 years of experience as a financier and company builder in the mining and oil and gas sectors. He co-founded and serves as chairman of B2Gold, a top-performing growing gold producer which is expected to achieve nearly 1 million ounces of low-cost gold production in 2018. He was also co-founder and chairman of Bankers Petroleum Ltd.; co-founder and chairman of Petrodorado Energy Ltd.; and until October 2007 was the non-executive chairman of Northern Orion Resources Inc. He also was previously the chairman and CEO of Yorkton Securities Inc., and a partner in investment banking with Gordon Capital Corp. in Toronto. Cross has an engineering degree from the University of Waterloo (1982) and received an MBA from Harvard in 1987.
Following a multi-million-dollar financing in Q1 2018, Standard Lithium is well-positioned to meet its upcoming milestones including two maiden resource reports and the launch of its breakthrough rapid lithium extraction technology.
StorEn Technologies Inc.

StorEn Technologies Inc. delivers proprietary vanadium flow batteries aimed at revolutionizing the world of residential and industrial energy storage. With an expected life of 25 years and more than 15,000 cycles, the company’s batteries satisfy market demand for efficient, durable and cost-effective energy storage, enabling self-consumption of self-produced electricity and the transition toward a carbon-free economy.
The company is currently accepting investments through a Reg A+ offering on StartEngine. For more information, view the company’s Offering Circular. To date, StorEn has raised more than $6.7 million from over 5,000 investors on the crowdfunding platform, along with venture capital from the ANYSEED Fund.
StorEn’s growing intellectual property portfolio currently features four international PCT patents and five trademarks, securing its innovative IP in all major regions and countries in the world.
A Disruptive Approach to Energy Storage
StorEn’s patent-pending all-vanadium flow battery technology offers a variety of benefits over existing lithium and lead acid batteries, including:
- Eco-Friendly: StorEn vanadium flow batteries are 100% recyclable, featuring a 100% reusable electrolyte and low GHGs emissions.
- Safe: The company’s batteries are both non-flammable and non-explosive.
- Cost Effective: StorEn’s cost/kWh is comparable to that of lithium batteries, but its cost/cycle is up to four times lower than lithium batteries, thanks to the exceptional duration of over 25 years or 15,000 cycles.
- Efficient: The company’s vanadium flow battery technology offers the highest power density thanks to MULTIGRIDS™, +35% in energy storage capacity with the same volume and +5% round-trip efficiency in harsh climate thanks to its proprietary THERMASTABLE™ geothermal design. StorEn’s solution is also virtually maintenance-free, leveraging its proprietary RESAFE™ and EQUILEVELS™ technologies.
StorEn batteries are modular and configurable in either 20kWh or 30kWh versions sharing the same Power Module, ensuring that customers only pay for the energy capacity they really need. The ability to connect additional modules allows for maximum flexibility.
Traction in the Market
To date, the total investment in the company’s technology has exceeded $2 million, and it is already putting these efforts to work. StorEn secured a $500,000 order in Australia to provide 30 kWh StorEn vanadium flow batteries to a renewable hydrogen plant at Queensland University of Technology (QUT), where researchers will develop safety standards for the future use of vanadium flow batteries. The first battery – the first of its kind in Australia – was installed in Brisbane in November 2020 at the National Battery Testing Centre (NBTC), a flagship project of the Future Battery Industries CRC. Additional units are being manufactured.
StorEn has also entered into a supply chain deal with Multicom Resources, an Australian mining company which is the owner of two vanadium mines. Through this agreement, StorEn has secured the exclusive availability of vanadium for up to 20 years with either a price cap or at market price, whichever is lower.
Capitalizing on the Australian government’s support to fulfil the country’s energy storage opportunity, Multicom’s subsidiary, Freedom Energy, has agreed to assemble StorEn batteries within Australia and distribute them widely across the wider Asia Pacific region. In addition to an initial pilot plant, Multicom has completed a concept design for a full-scale manufacturing facility for StorEn batteries.
Market Opportunity
The shift to renewable energy sources is on, with governments around the globe discussing and implementing initiatives to reduce dependence on fossil fuels. McKinsey & Company research suggests that, by 2035, more than 50% of global power generation will come from renewable sources.
Spurred on by this transition, demand for reliable energy storage systems is expected to attain exponential growth in the coming years, positively influencing the energy storage industry landscape, according to Grand View Research.
Data from Fortune Business Insights projects that the global battery energy storage market will reach $19.74 billion by 2027, recording a CAGR of 20.4% from 2020 to 2027. The research firm suggests that improving access to electricity across the globe will be a prominent trend shaping the growth trajectory of this market, which is particularly noteworthy for StorEn and its TITANstack™ grid-scale energy storage solution.
Over a billion people still do not have access to electricity. The electrification of these unserved communities can become a reality with mini grids, using solar plus energy storage. StorEn’s vanadium flow batteries could be a key technology toward providing universal access to affordable, longer lasting and dependable energy. In support this critical mission, StorEn Technologies is a member of the Alliance for Rural Electrification and the Global Off-Grid Lighting Association.
Management Team
StorEn is led by an executive team with decades of experience in the vanadium flow battery industry.
Founder Carlo Brovero has served as the company’s chief executive officer, treasurer and director since its inception in January 2017. From 2013 to 2019, Mr. Brovero served as a consultant for eCaral Ltd., a management consulting firm. From 2013 to 2015, he served as an advisory board member for Proxhima S.r.l., a vanadium flow battery company, which was sold to the Gala Group, a utility listed on the Milan Stock Exchange. From 2010 to 2016, Mr. Brovero served as International Sales and Marketing Director for iVis Technologies, the manufacturer of an excimer laser therapeutic and refractive platform for corneal surgery. He holds an MBA from Aston University in Birmingham, UK.
Founder Angelo D’Anzi has served as StorEn’s chief technology officer and director since the company’s inception. He is primarily responsible for the technical development of StorEn’s products. Since May 2018, Mr. D’Anzi has also served as a director of Arco Fuel Cells S.r.l., where he is responsible for the company’s fuel cell technical development activities. Mr. D’Anzi co-founded vanadium flow battery company Proxhima in 2013. In 2000, he founded ROEN-EST, a fuel cell company that was eventually acquired by the Morphic Group, a cleantech holding company listed on the Stockholm Stock Exchange. Mr. D’Anzi holds 14 international patents and received the 2003 Sapio Award in the Energy and Transportation category. He holds an MBA from the LUISS Business School in Rome.
Founder Gabriele Colombo has served as secretary of StorEn since its inception. Since 2012, he has also served in various roles ranging from regional manager to CEO with Leonardo Hispania S.A., a subsidiary of the Leonardo Group of Italy, an aerospace, defense and security conglomerate. Mr. Colombo co-founded vanadium flow battery company Proxhima in 2013. He holds an honors degree in computer engineering from the University of Pisa and a master’s degree in business leadership from the University of Genova.
Streamlytics

Streamlytics provides ethical, people-powered data, spanning millions of data points from today’s fastest growing communities across the United States. The company unlocks the power of actual data usage, reflective of how people create data today – simultaneously across all types of platforms, not by rigid panels or unethical tracking. By partnering with consumers across the nation, the company has gained unparalleled access to audiences’ and shoppers’ content consumption and purchasing patterns across Netflix, Google, Amazon and more.
Streamlytics’ first consumer facing data acquisition app allows African American consumers to own their data through a data license, value their data with its proprietary data valuation algorithm, and get fairly paid for their data. The result is ethical data transactions and unmatched insight into the decisions that consumers are making across platforms. The company’s data signals are not limited to purchase and content consumption. The breadth of activity spans fitness, health and universal mobility. The current archaic model of consumer data collection across many industries is to use second- or third-party assumptive data based on cookies or affinities, which has a high margin of error causing an enormous amount of waste in financial resources for client organizations. Streamlytics provides clear, accurate, full-spectrum data, delivering the true picture of a coveted consumer group’s activity across their digital footprint.
Since its founding, the company’s mission has been to disrupt the deceptive online data collection processes that have become commonplace. Streamlytics’ drive to prioritize consumer data collection transparency and ethics has led to tremendous growth. The company recently announced it had reached a milestone of more than a quarter-billion data points. Streamlytics’ impressive growth over the past year is largely due to expansion, adding platforms like Apple, Uber, Uber Eats, Postmates and others. The company’s patent-pending data standard, Universal Data Interchange Format (UDIF), powers the unification of cross-platform data sources and formats into a single unified data format. Streamlytics leads the industry in consumer data unification, which is increasingly valuable as companies look to navigate away from third-party data solutions and integrate ethical first-party data across corporate strategy, product innovation, artificial intelligence, marketing and more.
How it Works
Streamlytics unifies consumer data from today’s fastest growing communities across popular platforms spanning over 400 million data points. We ethically unlock the power of actual usage data (directly from the source) and help companies grow by enhancing their 1st-party data strategy across sales, marketing, product, and artificial intelligence.
Streamlytics data enhances existing measurement tools by focusing on density. The company’s approach provides a number of benefits over traditional data sourcing platforms, including:
- Multidimensional data that offers visibility into consumption behaviors that define decision drivers for consumers
- An integrated approach that connects a variety of data sources and types to paint the clearest picture of consumer behavior
- A clear understanding of the consumer, allowing for greater targeting precision that directly impacts the effectiveness of campaigns
- Ethical sourcing, with consumers directly compensated for their data
- Protection of all personal identifying information (PII) to ensure privacy and security
The company sells data that has been ethically sourced through a Standard Datastream (a streamlined feed consisting of roughly 22.5 million data points) and a Custom Datastream (a full spectrum feed spanning over 150 million data points). Client organizations subscribe to either datastream, based on the specific audiences they want to reach. Organizations most often use Streamlytics data to enhance their first party data strategies in an effort to increase revenue and sales, refine corporate strategy and enhance machine learning training data to reduce algorithmic bias.
Market Outlook
The global alternative data market was valued at $1.06 billion in 2019 and is expected to grow at a CAGR of 40.1% to reach more than $8 billion by 2027. The global artificial intelligence market was valued at $62.35 billion in 2020 and is expected to achieve a CAGR of 40.2% from 2021 to 2028, according to data from Grand View Research.
Streamlytics believes a new market space is emerging at the intersection of these two thriving industries called ‘Community Driven Data’, which will comprise consumers who have opted in to share their data, and companies that decide using ethically sourced data is better than fines and negative media coverage they could get from continuing to do it the old way.
Streamlytics has positioned itself as the leader of this emerging new market space as consumers increasingly opt out of sharing their data under the current model, and as new laws – like Prop 24, the California Consumer Personal Information initiative passed overwhelmingly by voters in 2020 – mandate greater privacy protections for, and limits on corporate use of, consumer data.
Management Team
Angela Benton is founder and CEO of Streamlytics. She is a pioneer of diversity in the technology industry and of raising awareness around the inequalities that exist in the industry. In 2011, she founded NewME, the first entrepreneurial accelerator globally for minorities. Through her leadership, NewME has accelerated hundreds of entrepreneurs, helping the nascent companies to raise more than $47 million in venture capital funding. That company was acquired in 2018.
Arisha Smith is the Chief Revenue Officer of Streamlytics. An innovator in advertising technology, she has designed growth strategies for businesses leveraging digital, social and mobile platforms for over 20 years. She has held marketing positions at Accenture and Microsoft, as well as at Vibe Media. She earned an MBA from Florida A&M University.
Sugarmade, Inc. (OTC: SGMD)
Sugarmade, Inc. (OTC: SGMD) is a product and brand marketing company investing in operations and technologies with disruptive potential. The company is focused on collaborating with real people in real-time to identify the emerging desires and behaviors poised to unlock new opportunities and pathways for growth. Sugarmade seeks to redefine the marketplace by nurturing an innovative and compelling relationship between brand, botany and business – resulting in both undeniable consumer value and an intriguing cross-pollination of revenue sources.
The company’s core strategic plan is centered on expanding its end-market access as a central player in the growing California cannabis delivery marketplace while developing its in-house cannabis production capacity to verticalize operations in the space. Through a combination of organic growth and strategic acquisitions, Sugarmade intends to develop a full farm-to-door vertically integrated cannabis business.
Brand Portfolio
Sugarmade has investments in a number of subsidiaries with active operations in the California cannabis sector. These include:
- NUG Avenue – Sugarmade owns a 70% stake in NUG Avenue, a cannabis delivery service based in Southern California providing hand-selected top-shelf products from Stiiizy, Kanha, PlugPlay and more.
- BudCars – Sugarmade is an investor in cannabis delivery service of BudCars’ first operating location in Sacramento, California. BudCars is an online-shopping experience designed to provide new customers with an easy way to discover and order cannabis products within minutes.
Acquisition of Lemon Glow Company
On May 17, 2021, Sugarmade took a major step toward closing the loop on what its management team believes to be one of the most promising vertically integrated cannabis models in the thriving California market when it announced the signing of a definitive agreement for its acquisition of Lemon Glow Company Inc.
The Lemon Glow acquisition includes 640 acres of property, 32 of which have already been designated for outdoor cannabis cultivation. Per the company’s news release, the annual potential cultivation yield at the property is estimated to be approximately 4,000 pounds of dry trimmed cannabis flower per acre per year, which represents approximately 128,000 pounds, or 64 tons, of dry trimmed cannabis flower per year in total.
Notably, Sugarmade also benefits from the acquisition in terms of team capital, as Lemon Glow executive team members will stay on and become the core management team at the cannabis cultivation site, granting the operation over 30 years of cannabis cultivation experience.
“The Lemon Glow team are tremendous additions to the Sugarmade team,” Jimmy Chan, CEO of Sugarmade, commented in announcing the definitive agreement. “They have vast experience and established skills, as well as intricate knowledge of the property and its local grow context. That’s an enormous added value proposition in this deal. We look forward to bringing them on board, ramping up operations at the property, and taking key steps toward delivering on the promise of Sugarmade’s farm-to-door vision.”
Market Opportunity
The California cannabis industry has continued to record tremendous growth since voters approved a measure to legalize recreational use of the plant in 2016. According to data from MJBizDaily, California’s legal market hit $4.4 billion in sales in 2020, up from $2.8 billion in 2019 and $1.4 billion in 2018.
Those figures highlight California’s status as the largest legal cannabis market in the world. With roughly 28 million residents over the age of 21, California is more than twice the combined size of the four states (Arizona, New Jersey, Montana and North Dakota) that legalized cannabis in 2020.
The COVID-19 pandemic was a key driver in the growth of cannabis delivery services throughout the state in 2020. One California cannabis delivery firm reported a 60% increase in new delivery customer sign-ups in the 30 days following the March 13, 2020, declaration of a national emergency. As a result of this boom, tech companies in cannabis ecommerce were able to dramatically increase their market share.
Sugarmade’s continued efforts to develop a farm-to-door vertically integrated cannabis business position it to capitalize on these trends as the California cannabis industry continues to expand moving forward.
Management
Jimmy Chan is the CEO of Sugarmade. He is an experienced business executive instrumental in growing multiple business operations with a strong expertise in international trade and banking, international manufacturing and importation. He is also the founder of CarryOutSupplies.com, a company that revolutionized the custom-printed paper supplies subsector of the quick service restaurant industry, which merged with Sugarmade in 2014.
Sustainable Green Team Ltd. (OTC: SGTM)
Sustainable Green Team Ltd. (OTC: SGTM) is currently incorporated and in good standing in the State of Delaware. The company conducts business activities principally through its two wholly owned subsidiaries, National Storm Recovery LLC, a Delaware limited liability company, and Mulch Manufacturing Inc., an Ohio corporation.
Through its subsidiaries, the company provides tree services, debris hauling, removal and biomass recycling, mulch manufacturing, mulch packaging, sales of next-generation mulch products and sawmill operations manufacturing specialty cypress lumber.
The company’s primary corporate objective is to provide a solution for the treatment and handling of tree debris that has been historically sent to local landfills and disposal sites, creating an environmental burden and pressure on disposal sites around the nation, by converting the biomass into a marketable beneficial use product.
The company’s solutions are founded in sustainability, based on vertical integration efforts that begin with collecting tree debris through its tree services division and collection sites and then, through its processing division, recycling and using that tree debris as a feedstock that is manufactured into a variety of organic, attractive, next-generation mulch products that are packaged and sold to landscapers, installers and garden centers.
The company plans to expand its operations through a combination of organic growth and strategic acquisitions that are both accretive to earnings and positioned for rapid growth from the resulting synergistic opportunities identified. The company’s customers include governmental, residential and commercial clients.
Company Products and Services
Tree Care, Removal and Services
National Storm Recovery LLC (“NSR LLC”), d.b.a. Central Florida Arborcare (“CFA”), was initially founded to provide tree maintenance, disaster recovery, debris hauling, removal and disposal services. Each of these services is provided to residential, commercial and governmental customers and was structured to drive revenue for the company. Examples include:
- A multi-year contract with the Town of Oakland, Florida (an area known for its large old oak trees), for emergency debris hauling and tree removal and
- A multi-year contract with the Orange County, Florida, School District (covering 267 properties that include schools, administrative sites and maintenance facilities) for tree removal, trimming and maintenance services.
In each case, these contracts are renewable following their initial multi-year terms, with aggregate terms of five years.
NSR LLC has spent years perfecting its technique for proper tree care, removal and services. From tree removal, stump grinding and tree care to grapple hauling and storm recovery, NSR LLC/CFA ensures properties remain safe and business can continue as usual.
NSR LLC/CFA was established as a company to provide tree maintenance, disaster recovery, debris hauling (Rose Transport), removal and disposal services – services that provide it with access to a large amount of wood or tree debris.
Thought of from a different perspective, the company has access to a large amount of “feedstock” that is required to manufacture wood-based mulch products. However, unlike traditional wood-based mulch manufacturers who purchase ALL their feedstock, the company is paid to cut it, paid to haul it and paid to dispose of large volumes of clean wood debris. Its cost, in that limited equation, was its own disposal cost. However, by processing the tree material into mulch and selling it, the company:
- Eliminates its disposal costs;
- Receives the feedstock it would need as a mulch manufacturer at a much lower net cost;
- Does not have to police its suppliers to ensure responsible tree harvesting, because the trees and material the company handles are either from trees and branches downed in storms or cut as part of the care and maintenance of the trees for which it is paid to care; and
- Has a “cost structure” for its feedstock that is even better than a competitor that secures feedstock using unscrupulous or irresponsible harvesting methods and/or sources.
So, by grinding, screening and packaging the tree material that it is already receiving (and is paid to receive), the company is able to leverage its existing activities, create additional value and position itself to substantially increase its overall revenue and earnings prospects – all while decreasing the burden that this material would otherwise place on local landfills or collection sites.
Mulch
In February 2020, SGTM acquired 35-year-old industry leader and innovator Mulch Manufacturing Inc. (“MMI”). Structured as a share exchange, this strategic partnership provides the company with a significantly larger footprint in the mulch industry.
MMI will strive to become more vertically integrated by receiving a substantial volume of wood fiber recovered from CFA’s operations and its Rose Transport Grapple fleet to feed the growing raw material needs to augment its long-standing fiber procurement process. MMI has the product line and distribution system to address a substantial customer base, which can be expanded. MMI offers many mulch variants, including cypress, cypress bark, pine bark, IPEMA certified playground chips, numerous colored mulches and its proprietary patented ‘SoftScape’.
The acquisition includes MMI’s national distribution agreements, an increase in production and packaging capacity and its sales contracts with numerous big box retailers. MMI includes mulch production, sawmill operation, Nature’s Reflections colorant manufacturing and equipment manufacturing.
Next-Generation Products
The company’s vision and commitment to the environment is paired with MMI’s revolutionary “next-generation” mulch product, Nature’s Reflections Softscape®.
Softscape mulch products, created from natural forest products, are color-enhanced with environmentally safe colorants to provide four-year color retention and are free from contaminants. Safe for people and pets, Softscape allows water and air to penetrate soil and roots, which is vital to plant health.
Cypress Sawmill
Mulch Manufacturing’s Homerville Sawmill Operation is located in Homerville, Georgia. The mill was founded in 1981.
The mill currently operates as the nation’s only mill to exclusively saw cypress and has capacity to saw 6.5 million board feet annually. The mill has a drying capacity of 3.1 million board feet annually, as well as milling capabilities, and it produces in excess of 2,500 trucks of mulch products annually.
The mill is sourced through a network of timber companies, timber dealers and private landowners. Mulch Manufacturing ensures that best management practices are employed when harvesting cypress logs to ensure the sustainability of the product.
Room for Expansion
The company has received final zoning approval for its 100-acre site, located in Lake County, Astatula, Florida, which will serve as the company’s flagship tree debris collection site. The facility will also house the company’s mulch manufacturing, soil composting and production bagging. This prime location includes a 5,000-square-foot building that contains warehouse and office space. The 100-acre property can accommodate millions of cubic yards of organic debris and will allow the company’s debris hauling division to realize significant savings on its transportation costs.
The company has chosen as its new headquarters the Mulch Manufacturing facility in Jacksonville, Florida, comprising a 100,000-square-foot warehouse/truck terminal. It is located on 26 acres for centralized operations of MMI and NSR LLC, and it has ample room to expand as needed. The facility recently began new operations to provide grapple truck services, wood/biomass recycling and operation of a new mulch production/bagging facility in the Jacksonville area.
Leadership
SGTM’s leadership team boasts more than 40 years of next-level experience with mulch manufacturing and treating and caring for trees. This team is guided by a roster of highly qualified professionals:
- Tony Raynor, Chief Executive Officer
- Edward Lee, Chief Operating Officer
- Scott Siefker, Chief Financial Officer
Sycamore Entertainment Group Inc. (OTC: SEGI)
Sycamore Entertainment Group Inc. (OTC: SEGI) is a diversified entertainment company specializing in the acquisition, marketing and worldwide distribution of quality finished feature-length motion pictures.
Through wholly owned subsidiary SEGI.TV, the company offers a streaming experience built on the pillars of equality, sustainability and community. SEGI.TV taps into the changing cultural environment, offering movies and television programming for a diverse audience – all without a subscription fee.
SEGI.TV
Launched in late 2020, SEGI.TV is scheduled to reach 100 million U.S. household televisions and 200 million mobile devices through Roku, Amazon Fire TV, Apple TV, Samsung Smart TV and others. The OTT streaming network operates via an ad-supported video on demand (AVOD) model, allowing Sycamore to control revenue growth by negotiating rates with advertisers as its userbase continues to expand.
The company expects this AVOD model to help SEGI.TV more efficiently grow its market share while avoiding direct competition with subscription service players such as Netflix and Hulu. Other industry players who have leveraged and grown through the ad-based revenue model include Tubi (33 million monthly users, acquired by Fox), YouTube (2 billion monthly users, acquired by Google) and Pluto TV (28 million monthly users, acquired by NBC/Viacom).
SEGI.TV lives up to its brand promise of inclusion, equality and community by:
Attracting Original/Hard-to-Find Content
SEGI.TV features uplifting content aimed at helping its users tap into the changing cultural environment, including movies, TV and sporting events, and the company continues to seek-out new and engaging programming.

On March 19, 2022, SEGI.TV streamed the long-awaited grudge match between Hafthor Bjornsson and Eddie Hall, former World’s Strongest Men. Deemed ‘The Heaviest Boxing Match in History’, the bout was available to watch in just two clicks for free, without any time-specific trials, paywalled content or sneaky subscriptions.
In speaking about the match, Sycamore CEO Edward Sylvan stated, “With live streaming sports revenues expected to quadruple by 2028, we at SEGI.TV are uniquely positioned to capitalize on this new ad supported model.”
Making Inroads with New Users
As consumers continue to shift away from traditional cable and satellite toward OTT streaming, existing options are divided into two primary categories – ad-supported and premium. While industry giants like Netflix, Disney and Apple target customers with non-ad-supported options, SEGI.TV’s AVOD model positions it as a solid alternative for individuals who are reluctant to commit to a subscription-based platform.
Increasing Viewership through Branding and Awareness
Sycamore has entered sponsorship agreements for multiple motorsports events in an effort to connect with global influencers and build brand awareness.

- SEGI.TV sponsored the No. 10 Dallara-Honda of Alex Palou when he took the checkered flag, winning the Honda Indy Grand Prix of Alabama at Barber Motorsports Park.
- SEGI.TV sponsored the No. 50 car of Floyd Mayweather’s TMT Racing for NASCAR’s Coca-Cola 600 at Charlotte Motor Speedway.
- SEGI.TV sponsored the No. 99 SEGI.TV GMC HUMMER EV.R in the Extreme E all-electric global racing series, which focuses on bringing awareness to environmental and equality issues worldwide.
- SEGI.TV has the North American broadcast right to the all-electric eSkootr Championship
Market Opportunity
The gradual move away from traditional cable and satellite TV subscriptions in the U.S. and around the world has created an opportunity for streaming companies to capitalize. According to data from Statista, the number of pay TV households in the U.S. declined from a peak of 100.5 million in 2014 to roughly 73 million in 2021. Likewise, pay TV revenue in the U.S. decreased from $104 billion in 2015 to $74 billion in 2021.
The loss for cable and satellite providers has corresponded with a boom for video-on-demand services. Subscription video-on-demand (SVOD) revenue in the U.S. reached $25 billion in 2021. According to Digital TV Research, AVOD revenues are predicted to more than double between 2018 and 2024 to reach $56 billion across 138 countries. Even SVOD mainstay Netflix is exploring AVOD as a way to combat declining subscription numbers.
As Sycamore continues to expand SEGI.TV with new, unique and eventually premium content, it is uniquely positioned to benefit from this industry trend. Through its FAST (Free Ad-Supported TV) channels, SEGI.TV positions Sycamore as one of only a handful of publicly traded, pure play companies operating in the space.
In an October 2021 report, Variety called FAST “the latest avenue for established media and entertainment companies,” and recent moves by entertainment mainstays support this notion. In April 2022, Amazon rebranded its FAST-focused AVOD service to Amazon Freevee and highlighted the rapid growth of the platform, which has tripled its monthly active users.
“Advertising video-on-demand (AVOD) and free ad-supported TV (FAST) channels are the biggest winners of the Streaming Wars, we believe, because ‘free’ always has the largest TAM (total addressable market),” Laura Martin, an analyst with equity research firm Needham, said in a recent note to clients.
Management Team
Edward Sylvan is the CEO and Co-Founder of Sycamore. He has more than 30 years’ experience in the financial service and banking industry. His banking expertise helped cultivate more than 20 years of entertainment industry relationships.
Terry Sylvan is the company’s CMO and Co-Founder. He brings to Sycamore more than 25 years of marketing and advertising experience, from global brand assignments to film marketing.
TAAT Lifestyle & Wellness Ltd. (CSE: TAAT) (OTCQX: TOBAF)
TAAT Lifestyle & Wellness Ltd. (CSE: TAAT) (OTCQX: TOBAF) is a life sciences company dedicated to giving legal-aged smokers the choice to keep the smoking experience that they enjoy with no nicotine and no tobacco.
The key players of TAAT Lifestyle & Wellness are from leading tobacco brands. They are guiding the mission with the company’s proprietary product, TAAT(TM), which uses the company’s proprietary Beyond Tobacco(TM) base material. The base material undergoes a 14-step process to taste and smell just like tobacco and uses a patent-pending refinement technique.
This provides the company with unique opportunities on the global tobacco market, which was estimated at $849 billion in 2019, with approximately 1.3 billion people using tobacco in some form worldwide (https://nnw.fm/bvKFL).
TAAT Lifestyle & Wellness was founded in 2006 and is headquartered in Vancouver, Canada, with operations in Las Vegas, Nevada.
TAAT(TM)
TAAT is a smokable alternative to tobacco cigarettes using the Beyond Tobacco base material, which contains zero tobacco and zero nicotine. The current TAAT offering comes in three varieties: Original, Smooth and Menthol, which were launched during Q4 2020 in Ohio. The company’s Ohio tobacco wholesaler also distributes for major tobacco industry names such as Altria, RJ Reynolds (a subsidiary of British American Tobacco) and ITG.
The TAAT Beyond Tobacco experience was created to replicate the sensory elements of smoking a tobacco cigarette. Market testing in California and Nevada reached a consensus that TAAT products offered no significant differences in experience when compared to tobacco cigarettes, in terms of the following aspects:
- Visual – the nearly identical product packaging and enhanced smoke volume
- Auditory – the “crackling” sound of the base material when it is ignited
- Smell – when burning, TAAT emits a tobacco-like scent
- Taste – the patent-pending Beyond Tobacco base material undergoes a refinement process that creates a tobacco-like taste
- Touch – TAAT satisfies the “hand-to-mouth” fixation and motor habits, such as flicking ashes
TAAT Beyond Tobacco Targeting Current Smokers
TAAT Lifestyle & Wellness is currently targeting the market of legal-aged smokers with its proprietary product. The company aims “not to create a new problem, but to solve an existing one.” TAAT Lifestyle & Wellness offers a non-addictive alternative to tobacco, with several competitive advantages making it a promising option on the United States market, such as:
- Price – TAAT can be offered at a lower price than competing products in the tobacco category, which adds to the propositioned value for current legal-aged smokers.
- Experience – TAAT appeals to current smokers who wish to give up the tobacco and nicotine but keep the smoking experience they enjoy.
- Branding/Packaging – TAAT is American-grown and American-made, with its Beyond Tobacco base material serving as a legacy to the combustible tobacco products.
The current alternatives to cigarette smoking do not offer a comparable experience. Previously marketed products, like vaping, proved difficult for some legal-aged smokers to adopt, as the experience was too different from traditional cigarettes.
Market Outlook
In 2016, the United States tobacco market was valued at over $100 billion, a number that’s expected to grow over the next decade (https://nnw.fm/yd8oP). In terms of volume, over 215 billion cigarettes were sold to roughly 34 million adults in the United States in 2018. These numbers represent almost 14% of the adult population. Of those, almost two-thirds smoked more than 15 cigarettes in one day. A standard pack is comprised of 20 cigarettes.
The company’s Beyond Tobacco, as a non-tobacco product, has a price-driven consumer advantage in many states. While state taxes on traditional cigarettes vary, most tend to average around $1.82 per pack. Washington D.C. is on the higher end of the tax spectrum at $4.50 per pack, whereas Missouri is only $0.17 per pack (https://nnw.fm/D3WnT).
TAAT Lifestyle & Wellness estimates that, if one pack of TAAT Beyond Tobacco was sold at 20% of all United States tobacco points of sale, the product would capture 0.25% of the market, the equivalent of approximately 2.7 million cartons of cigarettes per year.
Management Team
Setti Coscarella is the Chief Executive Officer of TAAT Lifestyle & Wellness Ltd. He is experienced in investment banking, private equity and entrepreneurship. In 2017, Mr. Coscarella was the lead strategist for Reduced-Risk Products at Philip Morris International. While there, he worked with thousands of smokers to better understand how to position smoking alternatives, developing programs that could help smokers convert to reduced-risk products. Mr. Coscarella holds an MBA from the Schulich School of Business, specializing in finance, marketing and corporate strategy. He also has a Bachelor of Science in mathematics and physics from the University of Toronto.
Tim Corkum is the company’s Chief Revenue Officer. He has a lengthy history in the tobacco industry, having served 21 years at Philip Morris International. Mr. Corkum has experience leading the international commercialization of combustible cigarettes and working on reduced-risk product offerings. During his 21-year tenure, he held senior positions in business development, sales strategy, key account management and corporate affairs. He holds a BA from Carleton University with a concentration in law.
Joe Deighan is Founder of TAAT Lifestyle & Wellness and oversees research and development. He is the founder of vape liquid ‘JJuice’, created in 2012. JJuice was distributed across all of the United States and in 26 other countries, alongside the private label production that was done for other brands. Mr. Deighan sold JJuice in a cash deal that was valued at over $800,000 in 2017. He currently handles all R&D and production for Beyond Tobacco, knowing the product better than anyone else in the company.
Founded in 2012, The Alkaline Water Company Inc. (NASDAQ: WTER) (CSE: WTER)
Founded in 2012, The Alkaline Water Company Inc. (NASDAQ: WTER) (CSE: WTER) is headquartered in Scottsdale, Arizona. Its flagship product, Alkaline88®, is a leading premier alkaline water brand available in bulk and single-serve sizes, along with eco-friendly aluminum packaging options. With its innovative, state-of-the-art proprietary electrolysis process, Alkaline88® delivers perfect 8.8 pH balanced alkaline drinking water with trace minerals and electrolytes and boasts the company’s trademarked label ‘Clean Beverage’. Quickly being recognized as a growing lifestyle brand, Alkaline88® launched A88 Infused™ in 2019 to meet consumer demand for flavor-infused products. A88 Infused™ flavored water is available in six unique all-natural flavors, with new flavors coming soon. Additionally, in 2020, the company launched the A88CBD™ brand, featuring a broad line of topical and ingestible products. These products are made with lab-tested full and broad-spectrum hemp and include salves, balms, lotions, essential oils, bath-salts, CBD infused drinks, tinctures, capsules, gummies and powder packs.
Innovation and Expansion
Founded in 2012, The Alkaline Water Company began with a mission to create the best-tasting water in the world. At the time, there were two emerging trends in health-conscious consumers: a growing interest in the alkaline diet and perceived health benefits of pink Himalayan rock salt. By combining these two concepts in an alkaline water and trademarking the name Alkaline88, The Alkaline Water Company began offering what it calls the smoothest tasting Clean Beverage™ in the U.S. enhanced-water category.
Now a top bulk alkaline-water brand (the company reported record sales in March and April 2020, surpassing March and April 2019 numbers by 114% and 171%, respectively), The Alkaline Water Company is committed to growing its national footprint through innovation and expansion. That mindset was evident as the company introduced eco-friendly aluminum bottles and branched out into flavor-infused waters; the company currently offers six different flavors: peach/mango, lemon/lime, raspberry, watermelon, blood orange and lemon.
The company’s commitment to innovation may be most evident in its newest product line: A88CBD. This line of CBD-infused products includes tinctures, capsules, gummies, salves, balms, hand and foot lotions, essential oils, bath bombs and bath salts, as well as CBD-infused drinks, water and beverage shots. These quality, CBD-infused offerings are all made with lab-tested, full-spectrum hemp and are conveniently packaged and perfect for on-the-go or at home use.
In addition, The Alkaline Water Company has implemented an aggressive growth strategy, with numerous organic initiatives focused on national multichannel, mass-market expansion through a direct-to-warehouse model and co-packing facilities that are strategically located within 600 miles of 95% of the U.S. population. In addition to this strong brick-and-mortar approach, the company recently launched a B2C e-commerce platform (www.A88CBD.com) and aggressive digital-marketing campaigns.
Clear Advantages in a Growing Market
With consistent growth year over year, the company reported $32.2 million in revenue in fiscal 2019 and has emerged as a growth leader in the functional (value-added) waters space, which is the fastest-growing segment of the bottled water industry.
The Alkaline Water Company’s efforts are focused on its clear competitive advantages, including its strong marketing (the inclusion of alkaline in product names); existing grocery channels, which feature excellent relationships and a nationwide broker network; distinctive branding; proprietary technology, which produces great-tasting, high-quality water, infused drinks and other products; and price, with a broad range of products in all formats, from bulk bottles to single serve.
As the company focuses on strategic growth, it is eyeing the impressive potential of a market that is on a strong upswing. Annual bottled water sales have now surpassed soda consumption, with soda sales in the United States having declined by $1.2 billion over the past five years. Some research indicates that the global bottled water market will reach an estimated $280 billion this year, while the CBD market is forecast to top $20 billion by 2024.
With its products available in all major trade channels, including grocery stores, drug stores, c-stores and big-box retailers, The Alkaline Water Company is also looking to expand into new spaces, such as health and beauty, hospitality and specialty retailer locations.
Seasoned Management Team
The Alkaline Water Company is led by an experienced team focused on the company’s core strategy of building a national retail footprint and extending its lifestyle brands into other consumer packaged goods categories.
Richard A. Wright, President, CEO and Co-Founder of The Alkaline Water Company Inc., oversees all aspects of the business, successfully guiding the company through strategic opportunities and delivering greater than 50% growth since the company’s inception. A passionate and versatile leader with a strong track record of innovation, collaboration and achieving goal-driven results, Wright is a serial entrepreneur with more than 41 years of experience. Early in his career, he spent years at one of the ‘Big Four’ accounting firms, working his way up to Regional Director of Tax and Financial Planning. As a CPA, entrepreneur and former CFO, Wright brings extensive knowledge of finance, operations, sales and marketing to the team, and he has participated in hundreds of M&A transactions throughout his career.
David Guarino, CFO, Secretary, Treasurer and Director, earned a Bachelor of Science in accounting and a Master of Accountancy from the University of Denver. From 2008 to 2013, Guarino was President and a Director of Kahala Corp., a worldwide franchisor of multiple quick-service restaurant brands with locations in 49 states and more than 25 countries. From 2014 to 2015, Guarino was President of HTI International Holdings Inc., a technology company focused on forward osmosis water filtration technology.
Frank Chessman, National Sales Manager, is a graduate of the University of Southern California’s Marshall School of Business. He spent 25 years with Ralph’s Grocery, Kroger’s largest division, working at many levels before ultimately becoming Vice President of Advertising & Marketing. He then served 14 years as Executive Vice President at Simon Marketing. Chessman has more than a decade of experience in the beverage manufacturing industry.
Brian Sudano, Director, is managing partner of Beverage Marketing Corporation and BMC Strategic Associates. Sudano’s experience covers nearly the entire beverage industry, from energy drinks to wine, with special expertise in beverage alcohol by virtue of varied industry experience across a broad range of projects. Sudano manages several major clients, providing ongoing strategic and market advice and leading projects in strategic planning, market entry analysis and planning, sales/distribution, business modeling, brand repositioning and international opportunity assessment. He has spoken at many beverage industry events and is a contributing editor at Beverage World magazine.
Aaron Keay, Chairman, has been a successful investor, entrepreneur and financier to multiple small cap and startup companies over the last decade. During his time with these companies, he served in advisor, board-member and senior-management roles. His experience ranges across multiple sectors in mining, biotech, health and wellness, tech and cannabis, where he has invested and raised more than $500 million.
Tingo Inc. (OTCQB: TMNA)
Tingo Inc. (OTCQB: TMNA) is a digital service agri-fintech technology company focused on foundation-level agriculture and related financial services in Africa. The company aims to be Africa’s leading agri-fintech player, transforming rural farming communities to connect through its proprietary platform to meet their complete needs – from inputs and agronomy to off take and marketplace – and deliver sustainable income in an impactful way. The company’s vision is to build complete digitally inclusive ecosystems that promote financial inclusion and deliver disruptive micro-finance solutions, empower societies, produce social upliftment in rural communities and open international opportunities.
Tingo believes that a truly connected world will help contribute to a better global society. The company’s core focus areas are telecoms, financial services/fintech and agritech. Tingo’s goal is to provide a best-in-class customer experience, support the domestic economies of its host countries and support technological and financial inclusion to end the poverty premium. Through this, Tingo hopes to deliver attractive returns to shareholders while investing in the long-term future of the company and its subsidiaries.
Global climate change is challenging sustainable production and food security. Tingo’s strategy and market execution provide an opportunity for Africa to be a core focal point to solve a number of key areas of concern, including food security, gender equality, financial inclusion and poverty alleviation, to name a few. Disruption of micro finance through the use of DeFi-based stable coins and smart contracts will give agri-communities access to capital markets-driven digital finance solutions that make them more competitive and sustainable economically, striking a good balance of returns between digital asset providers and Tingo as the service partner. This innovation will deliver significant access to much needed finance at ‘Grassroot’ levels, delivering tangible social upliftment and GDP growth in the African markets served by Tingo.
Tingo Mobile, with more than nine million subscribers, is Nigeria’s leading technology and device-as-a-service platform aimed at accelerating digital commerce, especially in the country’s agritech and fintech verticals. The company helps farmers acquire mobile phones through a unique leasing plan, connecting them to mobile and data networks through its own virtual mobile network. Tingo also connects farmers to markets, services and resources via Nwassa, its digital agritech marketplace platform that commenced operations in 2020. The company has also launched a beta version of TingoPay – a B2B and B2C fintech app aimed at providing financial services to users inside and outside of the agriculture value chain. Among the services offered are mobile wallets, payment processing and access to specialist lenders, insurers and pension products.
Tingo will soon announce its innovative blockchain-based solution for use of digital stable coins to empower frictionless trade across borders in Africa. The company’s market-proven model in Nigeria is its core foundation, enabling Tingo to deliver the same service model across Africa to become the continent’s leading agri-fintech business powered through smartphone technology.
The African Continental Free Trade (ACFT) plan will be a key framework to prepare the company to be the leading intra-Africa trading hub for trade flows across Africa in the medium term, when it is likely the agreement will be executed into tangible activity. Tingo is well positioned to easily transform the goals of the ACFT into reality when finally implemented by the African Union and the various African countries that have not signed up.
Tingo posted total revenue of $594 million in 2020, with $212 million EBITDA. As of December 31, 2020, Tingo has 9,344,000 subscribers. The company is confident that these figures will grow through its expansion across Africa and natural progression of business in Nigeria.
Businesses
Tingo has four core businesses:
- Mobile Phone Leasing – Tingo has distributed almost 30 million mobile handsets since 2014 and will continue to replace the devices of its installed customer base every three years. Tingo Mobile provides the latest mobile phone handsets at an affordable price point and allows customers to spread payments over 36 months.
- Mobile Voice and Data Service – Through a mobile virtual network, Tingo provides its customers with voice and data services, allowing customers to communicate effectively, both inside and outside the agricultural ecosystem.
- Nwassa Marketplace Platform – Nwassa is Tingo’s proprietary agritech platform which provides Africa’s farmers with access to global markets to secure more competitive pricing for their crops. The platform processes 500,000 daily transactions with a value of over $8 million. A select group of trusted partners can assist smallholder farmers and agricultural cooperatives with packaging, warehousing, and dry and wet cargo logistics, as well as up-to-date information from the global agricultural sector. Tingo provides its customers with digital wallet services, which enable them to send and receive domestic payments, monitor cash flow in real time and securely hold money. The company also provides access to other services, such as utility bill payment, virtual airtime top-up, insurance services and alternative lending solutions.
- TingoPay – Since the launch of the Nwassa platform, Tingo has been a dominant player in the B2B fintech vertical. After many successful months of operating Nwassa, Tingo entered the fintech B2C vertical to extend its B2B offering to a broader market beyond agriculture.
TingoPay is still in its beta phase and will launch in 2021 with a comprehensive marketing campaign. TingoPay offers the following services:
- Tingo Wallet top-up
- Peer to Peer payments, inclusive of merchant payments at the stores
- Utility payments – airtime, broadband, cable, electricity, water, hotel, flights etc.
- Pension payments
- QR code payment services
Market Opportunity
Africa is the second-largest continent by population. It is also the youngest by far, with a median age of 18 for its 1.3 billion people. Tingo believes the building blocks for growth in Africa’s agriculture industry are in place and that the company is well positioned to participate in the upside. Sub-Saharan Africa’s population is growing at a rate of 2.7 percent per year. At the current growth rate, the continent’s population will double by 2050. Africa’s youthfulness represents a significant opportunity for material growth in demand for agricultural commodities. This younger generation is also being born into a digital world and is comfortable using technology.
Africa’s governments are improving business conditions for entrepreneurs and small businesses. Sub-Saharan Africa’s World Bank Doing Business rank has improved from 45 in 2004 to 65 in 2020. Tingo believes this trend will continue and encourage establishment of more new ventures across all economic sectors, including agriculture.
Africa attracted $407 billion of Foreign Direct Investments (“FDI”) between 2014 and 2018. Investments are increasingly focused on services and industrial sectors. Only 20 percent of investments are in extractive industries – a clear reversal from 2008, when 55 percent of FDI was aimed at resource extraction. Tingo believes FDI into Africa will help resolve significant infrastructure constraints and create value for agribusiness.
Management Team
Dozy Mmobuosi is the CEO of Tingo. He cofounded Tingo Mobile PLC (Nigeria) in 2001 and led the design and launch of Nigeria’s first SMS banking solution, which is still in use in the country today. He also headed a team of more than 120 Chinese and Nigerian engineers in the construction of two mobile phone assembly plants in Nigeria, which have produced and distributed 20 million phones across the country. He has led Tingo’s growth to more than $600 million in revenue annually. He holds a Ph.D. in Rural Advancement from UPM Malaysia.
Dakshesh Patel is the CFO of Tingo. He was formerly CFO of NatWest’s Global Debt and Investment Banking division. He has served as a Director at Gerken Capital Associates, a San Francisco-based alternative asset fund manager. He also led the restructure of Lloyds Banking Group (last financial crisis); managed integration of two leading shipping groups’ global treasury function to create world-leading shipping group Maersk Shipping; built three fintech companies; and exited one to Worldpay. Mr. Patel has strong banking experience, with a focus on Africa. He is a chartered accountant.
Chris Cleverly is president of Tingo. He has served as CEO of the Made in Africa Foundation, and as CEO of blockchain payments gateway startup Kamari. He has been a board member of several companies, both public and private, in the UK, India, China and Africa. He has advised multiple UK companies on their entrance into African markets, and regularly advises the UK Government on development issues and African governments on investment issues.
Clarence Simms is the Chief Technology Officer at Tingo. He has 25 years of IT and IT management experience. He has worked in IT Shared Services Technical Operations and IT Program Management for Huawei Technologies and MTN. As an entrepreneur, he created Africaprepay.com, a service that allows African Diaspora travelers to send airtime, pay bills, send mobile money and transfer money to a bank account from anyplace in the world.
Rory Bowen is the Chief of Staff at Tingo. Mr. Bowen started his career in traditional capital and derivatives markets working for Moneycorp and Tradition UK in European and emerging markets across FX, interest rate derivative and government bond markets. He has also spent time with one of Europe’s fastest growing fintech’s banking circles. Before joining Tingo, he was Chief of Staff at FinTech Alliance, an organization established in partnership with the UK Government Department for International Trade to foster innovation, growth and foreign direct investment (FDI) in the financial services sector and facilitate greater public/private cooperation.
Tryp Therapeutics Inc. (CSE: TRYP) (OTCQB: TRYPF)
Tryp Therapeutics Inc. (CSE: TRYP) (OTCQB: TRYPF) is a pharmaceutical company focused on developing clinical-stage compounds for diseases with high unmet medical needs through accelerated regulatory pathways.
The company was founded in 2019 and is headquartered in San Diego, California.
Innovative Drug Pipeline
Tryp’s current focus is on advancing its two drug development platforms: its Psilocybin-for-Neuropsychiatric Disorders (PFN™) program targeting fibromyalgia, eating disorders and chronic pain conditions; and razoxane for soft tissue sarcomas. The company intends to explore opportunities to monetize these platforms after generating Phase 2b clinical data.
The company’s development plans cover three strategic initiatives:
- Develop: Tryp intends to utilize the FDA’s 505(b)(2) regulatory pathway with available third-party preclinical data to shorten the timelines and lower the cost of its development programs.
- Protect: Tryp plans to utilize regulatory exclusivity, patents, trade secrets and proprietary know-how to protect the commercial lifespan of its drug candidates.
- Monetize: Tryp intends to seek out licensing, acquisition and co-development opportunities for drug candidates following their Phase 2 stages of development.
PFN™ Program
Through its PFN™ program, the company is focused on developing psilocybin-based drug therapies for certain neuropsychiatric disorders that have distinct advantages over other drugs currently on the market or in development. These advantages include:
- Increased efficacy
- Natural blood-brain barrier penetration
- Enhanced safety and toxicity profiles
- Reduced risk of abuse
- Reduced risk of addiction
Tryp’s PFN™ program features its lead drug candidate, TRP-8802. The company’s initial indication for TRP-8802 is fibromyalgia.
Fibromyalgia is believed to be a neurosensory disorder characterized in part by abnormalities in pain processing by the central nervous system. The three drugs with FDA approval for the treatment of fibromyalgia are Pregabalin (Lyrica®), Duloxetine (Cymbalta®) and Milnacipran (Savella®), which are only effective for a portion of patients suffering from the condition.
Tryp plans to seek FDA approval to proceed directly to Phase 2 clinical trials evaluating TRP-8802 as a treatment for fibromyalgia based on existing preclinical and clinical data for the active pharmaceutical ingredients in TRP-8802.
Tryp’s pipeline of indications for TRP-8802 also includes eating disorders and certain forms of chronic pain. The company expects to initiate Phase 2a clinical trials in these areas in 2021.
Tryp recently partnered with Albany Molecular Research Inc. (“AMRI”) for the manufacture of the company’s synthetic psilocybin using proprietary methods. AMRI has initiated the process of manufacturing a 200g non-GMP demonstration batch of psilocybin and will produce a batch of GMP psilocybin in mid-2021. As the holder of the Drug Master File, Tryp expects to be the only U.S.-based manufacturer of synthetic psilocybin in the industry.
Razoxane
Tryp’s second drug candidate, TRP-1001 (razoxane), is being developed as a treatment for soft tissue sarcomas and has been evaluated in multiple Phase 2 clinical trials conducted by clinicians unaffiliated with Tryp. The company believes that existing clinical data regarding razoxane will likely allow TRP-1001 to be studied in a Phase 2 trial without the need for extensive preclinical or Phase 1 trials.
Sarcomas are rare tumors that are derived from connective tissues in the body and comprise 7% of all cancers in children. In 2018, an estimated 13,000 new cases of soft tissue sarcoma were diagnosed, with the tumors resulting in over 5,000 deaths during that year in the United States alone (https://ibn.fm/nWOGq).
Market Outlook
With its drug development programs targeting multiple indications, Tryp is well positioned to capitalize on growth opportunities spanning a range of therapeutic markets. The global oncology drugs market, in particular, represents a sizable opportunity.
In 2018, oncology indications accounted for 25% of all drug sales, representing approximately $151 billion in market revenues. By 2024, spending on oncology-targeted therapeutics is expected to top $200 billion and account for roughly 30% of total drug sales, according to a study by Cowen Equity Research (https://ibn.fm/9iZhM).
Valued at $764 million in 2020, the global fibromyalgia treatment market presents unique opportunities for development due to the limited number of approved therapies. With treatment trending upward, the market is expected to grow at a CAGR of 9.2% and reach $1.4 billion in value by 2027 (https://ibn.fm/G66e7).
Management Team
Greg McKee is the Chairman and CEO of Tryp Therapeutics. He has more than 20 years of life sciences management and venture investment experience that he brings to the company. Before taking his role at Tryp, he was the founder of Torrent Ventures, an early-stage digital health and medical technology venture fund. Mr. McKee also served as the CEO of CONNECT, the largest Southern California start-up accelerator. Before this, he was the chairman, president and CEO of then publicly traded Nventa Biopharmaceuticals, which successfully merged with Akela Pharma. Mr. McKee earned a B.A. in Economics from the University of Washington, an M.A. in International Studies from The Joseph H. Lauder Institute, and an MBA from the Wharton School at the University of Pennsylvania. He has been a member of the Young President’s Organization (YPO) since 2006.
James Gilligan, Ph.D., is the company’s President and Chief Science Officer. He has over 35 years of experience in the life sciences industry, including research and development, clinical development, international regulatory affairs and manufacturing. Before joining Tryp, Dr. Gilligan was the Co-Founder and Managing Partner of The Bracken Group, a life sciences consulting firm. He was also the Co-Founder of Unigene Laboratories, which develops technology for the recombinant manufacture of peptide hormones. Dr. Gilligan received his Ph.D. in Pharmacology from the University of Connecticut and a MSIB from Seton Hall University. He continued his post-graduate education at the Roche Institute of Molecular Biology.
Tom D’Orazio is the Chief Operating Officer of Tryp Therapeutics. He has extensive experience in leading the development and commercialization of vaccines, drugs, radiopharmaceuticals and biologics. His prior leadership experience has been in commercial planning, marketing, partnership and business development roles. He was formerly the CEO of ImmunoPrecise Antibodies Ltd. (NASDAQ: IPA), where he led the transition from a private company to a public one. He co-founded and served as CEO of Superna Life Sciences, a specialty-pharma company focusing on niche drugs for cancer patients in Canada. Mr. D’Orazio has an MBA from Vanderbilt University with a primary focus in both finance and marketing and a B.Sc. in chemistry from Loyola University of Chicago.
Luke Hayes is the company’s Chief Financial Officer. He has played an active role in the life science industry for over 20 years with technology transfer, venture capital and finance experience. His career started with business development for Dow Chemical (NYSE: DOW), with responsibility for pharmaceutical customers such as Eli Lilly and AbbVie. Mr. Hayes has spent more than a decade doing venture capital investing while supporting companies as a director and advisor. He earned a B.S. in Chemical Engineering from Brigham Young University and an MBA from the UCLA Anderson School of Management.
Trxade Group Inc. (NASDAQ: MEDS)
Trxade Group Inc. (NASDAQ: MEDS) is an integrated pharmaceutical services company that offers a unique combination of a web-based purchasing platform (www.trxade.com) for transactions between independent pharmacists and drug distributors (B2B); a network of pharmacies with E-Hub software; a mail order pharmacy; and warehouse and drug delivery services. This synergistic combination of product offerings and superior data analytics is poised to benefit all stakeholders and consumers within the pharmaceutical industry.
Trxade will leverage and scale its fully integrated model to execute the following growth strategies:
- Increase share of pharmacist drug purchasing
- Additional SKUs and expand product breath
- Partner with Specialty and International Mfg.
- Expand mail order licenses to all 50 states
- Scale Delivmeds for consumer delivery nationwide
- Integration with telemedicine
- M&A Opportunities within drug value chain
Founded in 2010 and headquartered in Tampa, Florida, Trxade’s overarching corporate strategy is to penetrate the existing retail independent pharmacy marketplace and diversify the company’s pharmaceutical mix with additional specialty and acute care products. Trxade is advancing on this mission by focusing on three key niches in the health care market.
Business-to-Business (B2B)
The $330 billion U.S. pharmaceutical industry is comprised of more than 65,000 pharmacy facilities and 1,500 state-licensed suppliers. Roughly 24,000 of these facilities are independent pharmacies, which collectively spend approximately $93 billion a year on branded and generic drugs.
Trxade targets these independent pharmacies, leveraging a robust, “E-Bay/Kayak-like” technology platform with optimum buyer/seller pricing algorithms, product availability, and predictive data analytics features.
Trxade currently serves and transacts with more than one-third (10,250) of these independent pharmacies and facilitates over $10 million of drug purchases a month!
Consumer
Trxade also targets the “consumer side” of the pharmaceutical industry, aiming to lower prescription drug costs by attacking the inefficient value chain; offering drug price transparency and efficient buying; and, delivering drugs DIRECT to independent pharmacists and consumers.
The company operates a full-service mail order pharmacy for U.S. consumers, as well as a mobile app called “Delivmeds” (http://www.delivmeds.com) which enables SAME DAY home delivery of dispensed prescriptions.
Retail
Trxade’s Managed Services Organization (“TrxadeMSO”) enables its member independent retail pharmacies to get patients, process orders, and deliver or ship prescriptions to patients. TrxadeMSO provides access to encompassing network of pharmacies through the E-Hub software, allowing for timely and comprehensive medication fulfillment.
These offerings ensure the best-suited pharmacy receives the patient’s information, thereby ensuring appropriate medication coverage based on the patient’s location, payor coverage, and medication access/inventory. This will save the clinicians and their staff time as they benefit from efficiency and enhanced workflow management in script processing and fulfillment.
Health Care Market
The U.S. health care market currently hovers near $4 trillion and is expected to grow as the general population ages. This growth will have greater impact on consumers as out-of-pocket expenses also rise. Additionally, drug costs are paced to increase faster than the overall health care and well above inflation.
Drug pricing is variable, and reimbursement is squeezing profits. This provides significant opportunity for the Trxade model of price visibility and profit optimization.
Trxade’s fair online market platform targets the nation’s retail community and independent pharmacies, of which there are approximately 24,000 nationwide. TRxADE has found that independent pharmacies, in order to be cost-effective, often operate with minimal staff and conduct up-to-the minute price checks. The TRxADE S2P platform gives these pharmacists the ability to easily compare the price of drugs offered by various suppliers and select the most favorable deals, saving money by taking advantage of best purchase pricing.
TRxADE’s programs include:
- TRxADE Exchange, which opens and widens the distribution channel to the retail, community pharmacy. A purchasing pharmacy can view products from manufacturers, buying groups, and wholesalers on a real-time and continuous basis. This approach significantly enhances the competitive spirit of the exchange where the lowest price exists for each product at any given point in time. TRxADE has become a competitive tool for all progressive entities and is recognized for its easy searching of hard-to-find generic pharmaceuticals at substantially reduced prices.
- RX Guru™ is an industry-leading price prediction model that integrates product shortage insight into pharmacy acquisition benchmarks (“PAC”) to ascertain trends and pricing variances that result in significant purchasing opportunities. RX Guru affords members the opportunity to continuously benefit from real price purchasing opportunities that are concealed from the rest of the industry.
- Product Shortage Database – TRxADE maintains the most comprehensive retail, specialty and acute care pharmaceutical product shortage database in the country. Other industry competitors mainly restrict their efforts to specialty and acute care product shortages and narrowly research oral generic products. TRxADE’s advanced prediction tools help members source those hard-to-find products at affordable costs in a timely and easy-to-search process.
Management Team
Trxade’s management team is rich in expertise within the pharmaceutical supply chain and is supported by a base of advisors and contractors who are experts in related fields of the pharmaceutical sector.
Suren Ajjarapu – Chairman of the Board, Chief Executive Officer and Secretary
Suren Ajjarapu has served as Trxade’s chairman of the board, CEO and secretary since 2014, and as the chairman of the board, chief executive officer and secretary of Trxade Nevada since its inception. Ajjarapu also serves as a chairman of the board for Feeder Creek Group Inc., since March 2018. Ajjarapu formerly was a founder, CEO and chairman of Sansur Renewable Energy Inc., a company involved in developing wind power sites in the Midwest, United States; a founder, president and director of Aemetis Inc., a biofuels company (AMTX.OB); a founder, chairman and CEO of International Biofuels, a subsidiary of Aemetis Inc.; and a co-founder, COO, and director at Global Information Technology Inc., an IT outsourcing and systems design company. Ajjarapu holds an M.S. in environmental engineering from South Dakota State University, Brookings, South Dakota, and an MBA from the University of South Florida, specializing in international finance and management. Ajjarapu is also a graduate of the Venture Capital and Private Equity program at Harvard University.
Prashant Patel – Director, President and Chief Operating Officer
Prashant Patel has served as Trxade’s full-time president and COO, and as a director since the company’s acquisition of Trxade Nevada in 2014, and as the COO and president and as a director of Trxade Nevada since its inception. He has been a president and member of the board of Trxade since August 2010. Patel is a registered pharmacist and pharmaceutical consultant with over 10 years of experience in retail pharmacy and pharmaceutical logistics. He is the founder of several pharmacies in the Tampa Bay area, in Florida. Since 2008, Patel has been managing member of the APAA LLC pharmacy. Since 2007, Patel has been a vice president of Holiday Pharmacy Inc. Patel graduated from Nottingham University School of Pharmacy and practiced in the United Kingdom before obtaining his masters in Transport, Trade and Finance from Cass Business School, City University, UK.
Uranium Energy Corp. (NYSE American: UEC)
Uranium Energy Corp. (NYSE American: UEC) is a U.S.-based uranium mining and exploration company that controls one of the country’s largest historical uranium exploration and development databases. Founded in 2003, UEC is headquartered in Corpus Christi, Texas. Properties acquired by the company are primarily located within the United States, including Texas, New Mexico, Colorado, Arizona and Wyoming.
Through the use of historical exploration data, UEC has been able to target and acquire properties that have already been subject to exploration and development by senior energy firms in the past.
UEC is well-financed to aggressively pursue key developmental targets. The company is also well-positioned to capitalize on rising global demand for more uranium and more carbon-free energy, and it uses technology that contributes to a cleaner environment.
In-Situ Recovery (ISR) Technology
In-situ recovery (ISR) technology is a low-cost and environmentally friendly mining technology utilized by UEC at its fully licensed projects, including Palangana, Burke Hollow, Goliad and Reno Creek.
ISR technology involves the circulation of naturally occurring and benign groundwater through a uranium ore body. This natural water (that is unfit for any other use) plus oxygen is pumped into injection wells through the uranium ore body, where the uranium in the host sandstone is oxidized and solubilized. The uranium bearing groundwater continues to flow through the sandstone to the extraction wells, where it is pumped to the surface. This water proceeds to an ion exchange unit (like a big water-softener) for uranium removal, then is pumped back to the wellfield and again re-circulated through the ore body. This recirculation of the same groundwater continues over and over, until the uranium in the sandstone is depleted.
In the ion exchange process, the extracted uranium in solution is concentrated on resin beads for transport to the Hobson Processing Facility. There, the uranium then undergoes several simple processing steps before being dried and packaged as “yellowcake” that will be transported to a conversion facility, where its sold to UEC customers.
Hobson Processing Plant
Hobson is the centerpiece in UEC’s hub and spoke production strategy, with low-cost satellite ISR operations all within relatively short trucking distance. The plant is fully licensed and currently on standby with an annual production capacity of 2 million pounds of U3O8. The spokes of the UEC strategy include the Palangana, Burke Hollow, Goliad, Salvo and Longhorn ISR projects. With an improvement in uranium prices that justify production, UEC plans to restart the plant with uranium loaded resins originating first from Palangana and then followed by Burke Hollow. UEC has applied for a license amendment with the Texas Commission on Environmental Quality to increase the Hobson facility’s production capacity to 4 million pounds per year.
Current Projects
Uranium Energy’s current project portfolio includes:
- Texas – Hobson Processing Plant, Palangana Mine, Goliad, Burke Hollow, Salvo and Longhorn
- Wyoming – Reno Creek
- Paraguay – Oviedo, Yuty and Alto Paraná
- New Mexico – Dalton Pass and C de Baca
- Colorado – Long Park and Slick Rock
- Arizona – Anderson, Los Cuatros and Workman Creek
- Canada – Diabase
Uranium Market Outlook
The long-term fundamentals underlying the market continue to strengthen. Currently, UEC sees an annual gap of about 40 million pounds between uranium production and utility requirements. Current forecasts show this structural deficit persisting at least through 2026 and then expanding further to almost 70 million pounds per year by 2030. While secondary supplies have been filling the void, those supplies are not a sustainable long term supply source. There are different estimates on timing, but it is clear secondary supply (that includes inventory drawdowns) will be insufficient to fill the projected gap between supply and demand, and new production will be required. As this transition evolves, the market will become more production cost driven as opposed to inventory driven.
Higher priced contracts that have supported high production costs are continuing to roll out of producer and utility supply portfolios. These higher priced contracts are not replaceable, with current market prices below production costs for the vast majority of western producers. This will likely continue the trend of production cuts and deferrals until prices rise sufficiently to sustain long-term mining operations.
In the U.S., some of the foreign State-Owned Enterprise (“SOE”) supply that has been flooding the market will be reduced. Last year, the U.S. Department of Commerce negotiated an amendment to the Agreement Suspending the Antidumping Investigation on Uranium from the Russian Federation that reduces America’s dependence on Russian natural uranium concentrates by up to 75% from prior levels. Due to a prolonged weak pricing environment from an influx of price insensitive supply from SOEs, U.S. production is effectively zero, less than 1% of U.S. requirements.
On the demand side of the equation, further upside market pressure also appears likely to evolve as utilities return to a longer-term contracting cycle to replace expiring contracts. Over the longer term, there continues to be underlying and increasing demand building, as the globe continues a push toward carbon-free energy goals. Those goals will require the 24/7, base load, clean energy that nuclear power provides as part of the overall supply mix. A good example of that policy messaging came from Japan’s energy minister, who recently said he considers nuclear energy “indispensable” if the country is to meet its net-zero carbon emission goals.
Exacerbating the overall supply picture, lead times for new production typically range from seven to 10 years or longer. The market appears to be within the time frames required for investment to bring new supply online to meet those lead times. However, prices are not yet at levels that incentivize future production, increasing the probability of the potential for less supply than the market is currently pricing in. All things considered, UEC believes the supply and demand fundamentals should continue to exert upward pressure on uranium prices.
Management Team
Spencer Abraham is Chairman of the Board for UEC. He served as the 10th U.S. Secretary of Energy from 2001 to 2005. He is an honors graduate of Michigan State University and Harvard Law School, and he was a law professor at the Thomas M. Cooley School of Law. He was elected chairman of the Michigan Republican Party in 1983 and later served as deputy chief of staff in the office of the vice president and as co-chairman of the National Republican Congressional Committee. In 1994, Mr. Abraham was elected to the United States Senate from Michigan and has also served as a director of Occidental Petroleum and as the non-executive chairman of AREVA’s U.S. board.
Amir Adnani is the Chief Executive Officer, President and Director of Uranium Energy. He advanced the company from concept to United States production within its first five years. Mr. Adnani has developed an extensive pipeline of low-cost and near-term production projects. He is the founder and Chairman of GoldMining Inc. (TSX: GOLD) (OTCQX: GLDLF), a gold-resources acquisition and development firm. He is also the Chairman of Uranium Royalty Corp. (TSX.V: URC). Mr. Adnani holds a Bachelor of Science from the University of British Columbia. He is a director of the University’s Alumni Association.
Scott Melbye is the company’s Executive Vice President. He is a 36-year veteran of the nuclear energy industry and has held numerous leadership positions in major uranium mining firms. He is also the current President, CEO and Director of Uranium Royalty Corp. He is an advisor to the Nuclear Energy Program at the Colorado School of Mines. Prior to his work at Uranium Participation Corp., Mr. Melbye worked for Cameco Inc. for 22 years. He received a Bachelor of Science in Business Administration with a specialization in International Business from Arizona State University in 1984.
Bruce Nicholson is the company’s Vice President of Corporate Development. He has spent 16 years as a specialist in the industry, serving major United States and European banks, broker-dealers and investment funds. Mr. Nicholson is a member of the Minerals Economics and Management Society, Minerals Industry Analyst Group, and the New York Society of Securities Analysts. He graduated with an MBA in Finance from Rutgers University in 1995 and is a CFA charter holder.
VistaGen Therapeutics Inc. (NASDAQ: VTGN)
VistaGen Therapeutics Inc. (NASDAQ: VTGN) is a biopharmaceutical company committed to developing and commercializing a new generation of medications that go beyond the standard of care for anxiety, depression and other central nervous system (CNS) disorders.
The company is headquartered in South San Francisco, California, the “Birthplace of Biotechnology,” among the largest cluster of biotechnology companies in the world.
New Generation Medications
VistaGen currently has three innovative CNS drug candidates in its pipeline: PH94B, PH10 and AV-101. With a differentiated mechanism of action and an exceptional safety profile in all clinical studies to date, each of VistaGen’s three drug candidates offers significant commercialization potential in multiple large CNS markets.
PH94B
Fast-acting (10-15 minutes), non-systemic and non-sedating in Phase 2 clinical studies, PH94B is a first-in-class neuroactive nasal spray that, administered in microgram doses, binds to chemosensory receptors in the nasal passage that trigger neural circuits responsible for suppressing fear and anxiety caused by stressful social or performance situations.
PH94B is currently being developed as an acute treatment of anxiety in adults with Social Anxiety Disorder (SAD). In December 2019, PH94B became the first drug candidate to be granted Fast Track designation by the U.S. Food and Drug Administration (FDA) for development of a treatment for SAD, positioning it to potentially become the first FDA-approved fast-acting acute treatment for adults with the anxiety disorder, if planned Phase 3 studies are successful.
A successful Phase 2 program has been completed, and, after achieving consensus with the FDA in mid-2020 that the design of its Phase 3 studies of PH94B in SAD may mirror the design of the highly statistically significant (p=0.002) Phase 2 public speaking study of PH94B in SAD, the company’s preparations for pivotal Phase 3 clinical development of PH94B are underway.
To support Phase 3 development and commercialization of PH94B for anxiety disorders in large anxiety disorder markets in Asia, VistaGen recently entered into a strategic licensing and collaboration agreement with EverInsight Therapeutics, a company formed and currently funded by a large global venture capital firm, CBC Group. The company received a $5 million non-dilutive upfront license payment from EverInsight in August 2020. If Phase 3 development is successful, VistaGen is eligible to receive additional development and commercial milestone payments of up to $172 million, plus tiered royalties on sales of PH94B in Greater China, South Korea and Southeast Asia. VistaGen retains exclusive rights to develop and commercialize PH94B in all other markets.
VistaGen is also assessing potential Phase 2A clinical development opportunities to evaluate PH94B in a range of other anxiety disorders, including:
- Adjustment Disorder with Anxiety
- Generalized Anxiety Disorder
- Postpartum Anxiety
- Perioperative Anxiety
- Panic Disorder
- PTSD
PH10
PH10 is an investigational fast-acting synthetic neuroactive nasal spray with therapeutic potential in a wide range of neuropsychiatric indications involving depression and suicidal ideation. VistaGen is initially developing PH10 as a potential fast-acting, non-sedating, non-addictive new generation treatment of major depressive disorder (MDD).
Upon self-administration, a microgram-level dose of PH10 sprayed into the nose binds to nasal chemosensory receptors that, in turn, activate neural circuits in the brain that lead to rapid-onset antidepressant effects, without side effects, systemic exposure or safety concerns that may be caused by FDA-approved drug treatments for MDD, including oral antidepressants and intranasal esketamine.
In a published exploratory Phase 2A MDD study, PH10 demonstrated rapid-onset and sustained antidepressant effects without the serious psychological side effects and safety concerns of ketamine-based therapy.
Following successfully completed Phase 2A development of PH10 for MDD, the company is currently preparing for a Phase 2B program in MDD.
VistaGen is also assessing the potential for Phase 2A clinical development of PH10 in a range of other depression-related indications, including:
- Postpartum Depression
- Treatment-resistant Depression
- Suicidal Ideation
AV-101
Part of a class of new generation investigational medicine in neurology and neuropsychiatry known as N-methyl-D-aspartate receptor (NMDAR) modulators, AV-101 is an oral prodrug of 7-chloro-kynurenic acid (7-Cl-KYNA), a potent and selective NMDAR glycine site antagonist. This drug candidate has the potential to serve as an innovative treatment for MDD and multiple neurological indications where current therapies are unsatisfactory.
VistaGen is currently evaluating AV-101, in combination with FDA-approved probenecid, in a range of neuropsychiatric and neurological indications, with both MDD and Neuropathic Pain already granted Fast Track designation by the FDA. The company is assessing the combination for a potential Phase 1B study to support a potential Phase 2A program in one or more of the following indications:
- Major Depressive Disorder
- Neuropathic Pain
- Levodopa-induced dyskinesia associated with Parkinson’s disease therapy
- Epilepsy
- Suicidal Ideation
CNS Therapeutics Market Outlook
The global CNS therapeutics market is estimated to reach $130 billion by 2025. The market was valued at approximately $82.3 billion in 2017 and is anticipated to grow at a healthy CAGR of more than 5.93% from 2018 to 2025. Even before the onset of the anxiety- and depression-provoking stressors from the COVID-19 pandemic, this growth was expected to be driven by a rise in mental illnesses and increased awareness of psychiatric disorders (https://nnw.fm/K2m0s) – all likely to be amplified by the diverse impacts of the pandemic.
The two most common mental health conditions – anxiety and depression – cost the global economy an estimated $1 trillion each year. The impact of these conditions is particularly devastating among the young. Industry data suggest that approximately 20% of the world’s children and teens are affected by mental health conditions, and suicide is the leading cause of death among 15- to 29-year-olds (https://nnw.fm/oftNb).
VistaGen’s mission is to help address the unmet needs of patients suffering from CNS disorders whose current treatments are either inadequate or generate debilitating side effects and serious safety concerns, including risk of abuse and death.
“Now more than ever, the new generation anti-anxiety and antidepressant medications we are developing at VistaGen – PH94B, PH10 and AV-101 – are relevant, necessary and demand the highly-focused and passionate efforts of our team and partners, with the support of our stockholders, to advance them to patients whose lives are disrupted by anxiety and depression disorders,” VistaGen CEO and Director Shawn K. Singh said in his closing remarks at the company’s 2020 Annual Meeting of stockholders.
Management Team
Shawn K. Singh, J.D. is the Chief Executive Officer and a Director of VistaGen. He has served on the company’s board of directors since 2000. He has nearly 30 years of experience serving in numerous senior management roles across multiple industries, including private and public biotechnology, pharmaceuticals, medical devices, venture capital, contract research and development, and law. Singh has a B.A. with honors from the University of California – Berkley. He has a J.D. degree from the University of Maryland Carey School of Law. He is also a member of the State Bar of California.
H. Ralph Snodgrass, Ph.D., is the Founder, Chief Scientific Officer and Director of the company. Snodgrass has more than 20 years of experience in the biotechnology field as a senior manager. He is recognized as an expert in stem cell biology, with over 28 years of experience using stem cells as biological research tools to promote development and drug discovery. He received a Ph.D. in immunology from the University of Pennsylvania. Snodgrass has published over 50 scientific papers with more than 17 patents and a number of patent applications.
Mark A. Smith, M.D., Ph.D., is VistaGen’s Chief Medical Officer He has over 20 years of pharmaceutical industry experience, primarily with CNS drug development. Smith has been a successful leader in the discovery and development of approximately 20 investigational new drugs. He has been a part of numerous CNS-related clinical trials. Smith received a bachelor’s and Master of Science from Yale University and a Doctor of Medicine and Doctor of Philosophy in Physiology and Pharmacology from the University of California – San Diego. He completed his residency in the psychiatry department at Duke University Medical Center.
Jerrold D. Dotson, CPA, is the Vice President, Chief Financial Officer and Secretary of VistaGen. He has over 25 years of experience in senior management positions in finance and administration at both public and private companies. Dotson is a licensed CPA in California and received his B.S. degree (Cum Laude) in business administration with a concentration in accounting from Abilene Christian College.
Mark A. McPartland is the company’s Vice President of Corporate Development and Investor Relations. He has over 20 years of experience in senior management roles in corporate development and investor relations at both public and private companies. McPartland received his Bachelor’s in business administration and marketing from Coastal Carolina University.
Vivos Therapeutics Inc. (NASDAQ: VVOS)
Headquartered in Denver, Colorado, Vivos Therapeutics Inc. (NASDAQ: VVOS) is an emerging global leader in the treatment of mild-to-moderate obstructive sleep apnea (OSA), a debilitating condition affecting nearly 1 billion people worldwide. The company utilizes proprietary, ground-breaking technology, a proven go-to-market strategy, and a powerful executive team dedicated to changing the face of health care by helping people of all ages properly breathe and sleep.
At the core of Vivos’ mission to rid the world of mild-to-moderate OSA is the Vivos System®, a revolutionary clinical breakthrough in the treatment of mild-to-moderate sleep apnea often caused by craniofacial anatomy development. The Vivos System® multidisciplinary treatment protocol involves collaboration between physicians, specially-trained dentists who have completed advanced training in craniofacial sleep medicine, and other ancillary health care providers.
In support of its growth strategy, Vivos has established contract manufacturing facilities in the U.S., Canada and Asia.
Market & Technology Overview
Craniofacial developmental deficiencies, such as underdeveloped upper and lower jaws, are among the leading causes of OSA. According to a 2019 analysis from researchers at the University of California, San Diego, an estimated 81 million adults in North and South America suffer from moderate to severe OSA. The United States has the highest amount of these patients, with approximately 54 million adults affected, according to the report.
Registered with the FDA as a Specification Developer, Vivos develops and markets a number of oral appliances. Its technology represents the first non-surgical, non-invasive and cost-effective treatment for the estimated hundreds of millions of people globally who suffer from mild-to-moderate OSA.
Vivos integrates its specially designed, customized appliances into a patient-specific, multi-disciplinary clinical protocol, giving trained dental and medical providers the tools and roadmap needed to address certain craniofacial conditions that studies have shown to be associated with sleep-disordered breathing—including mild-to-moderate OSA.
The system’s treatment protocol involves collaboration between physicians, specially trained dentists who have received advanced training in craniofacial sleep medicine, and additional health care providers. Vivos-trained clinicians can be found in almost every major city in the U.S. and in many countries throughout the world. The company’s oral appliances have shown to be effective in over 15,000 patients successfully treated worldwide by approximately 1,200 trained dentists.
A New Paradigm in Sleep Medicine
Vivos’ proprietary system poses the potential to be the biggest breakthrough in the treatment of mild-to-moderate OSA since CPAP.
The Vivos System has been specifically designed to promote the proper growth and development of the hard and soft tissues surrounding and comprising the oral cavity, nasal cavity, upper and lower jaws, and other tissues which together form and shape the upper airway. As these areas develop more fully using the Vivos System, a patient’s airway typically widens and expands, enabling them to breathe properly through their nose. With a more open and less obstructed airway, and easier nocturnal breathing, the symptoms of SDB tend to diminish over time, and patients often report they no longer suffer from the adverse effects of SDB or OSA.
Use of the Vivos System is variable and case dependent, but typically recommended to be worn daily for 12 to 16 hours starting in the early evening and continuing overnight. The total treatment time typically ranges from 12 to 24 months, with 18 months being the approximate mean treatment time.
Biomimetic Oral Appliance

Vivos Therapeutics believes that the Vivos System technology represents the first non-surgical, non-invasive and cost-effective treatment focr people with mild-to-moderate OSA.
Combining technologies and protocols that can alter the size, shape and position of the tissues of a patient’s upper airway, the Vivos System opens airway space and can significantly reduce symptoms and conditions associated with mild-to-moderate OSA.
The Vivos System treatment is typically less than $10,000 and is often reimbursable by medical insurance as an out-of-network benefit.
A potentially serious medical problem with an alternative treatment therapy available in the dental office.
Hard and soft tissues of the craniofacial complex can be non-surgically enhanced using the proprietary Vivos® System devices and clinical protocols.
Leadership
R. Kirk Huntsman – CEO, Director
With experience in strategic development, technology acquisition and product planning, key talent recruitment, and target market prioritization, Huntsman brings a broad vision paired with leadership and strategic planning skills. He has significant start-up experience in a diverse range of market sectors, including medical devices, dental management, dental practice valuations and transitions, multi-location retail, financial and capital formation, consulting, outsourced services, imports and exports (China), medical services, and software and technology.
Dr. Dave Singh – Founder, Director
A doctor three times over in dental medicine, craniofacial development, and orthodontics, Dr. Singh was educated primarily in England and has lectured in North America, Europe, Asia, and Africa. The Global Summits Institute recently named Dr. Singh as one of the Top 100 Doctors in Dentistry.
Wrap Technologies Inc. (NASDAQ: WRAP)
Wrap Technologies Inc. (NASDAQ: WRAP) is an innovator of modern policing solutions. The company’s BolaWrap® product is a patented, hand-held remote restraint device that discharges an eight-foot bola style Kevlar® tether to restrain an individual at a range of 10-25 feet. Developed by award-winning inventor Elwood Norris, the company’s chief technology officer, the small-but-powerful BolaWrap assists law enforcement in safely and effectively controlling encounters, especially those involving an individual experiencing a mental crisis.
Non-Lethal Weapons Market Potential
The BolaWrap Remote Restraint device is an innovative police solution, designed to provide law enforcement with a unique mobile and humane restraint option that does not inflict pain and enables subjects to be detained from a distance without the use of force.
In 2015, the 10 cities with the largest police departments in the United States paid out a cumulative $248.7 million in settlements and court judgements in police misconduct cases, marking a 48% increase from the $168.3 million in 2010 (http://nnw.fm/ri0L9). The majority of these cases have centered around the improper use of force by law enforcement when subjugating individuals, with 25% of all fatal shootings by law enforcement in the United States reportedly involving mentally ill individuals who are often incapable of comprehending officer commands (http://nnw.fm/YVm8P). Moreover, the use of alternate devices has failed to produce the desired outcomes, with the use of tasers by police resulting in over 1,080 fatalities since 2000 (http://nnw.fm/2Nb1A).
This, in turn, has led to a greater demand for humane tools which are not reliant on pain compliance to subdue subjects. Since its IPO in December 2017, Wrap Technologies has enjoyed a spectacular rise in prominence. The company began field testing the BolaWrap product in July 2018, with the first international order received only a month later, in August 2018. By December 2018, the company had been uplisted to the Nasdaq Capital Market with over 1,000 shareholders – a significant increase from the 50 shareholders who had participated in the IPO just 12 months prior. Recently, the company has sought to increase its commerciality and product monetization, appointing Tom Smith, the founder of TASER International (now Axon, NASDAQ: AAXN), as its president in March 2019.
At present, over 140 police departments throughout the United States are actively carrying the BolaWrap, while over 1,700 police departments across the nation have reached out to the company to request BolaWrap demonstrations, training and quotes. BolaWrap has also been successfully marketed internationally and has been shipped to 19 countries thus far.
As of today, Wrap Technologies has built a network of 11 distributors across 45 states in the United States who are actively marketing the product to the over 900,000 active police officers in the country. In addition, the company now has a network of 15 international distributors based in 26 countries – with over 600 international requests received thus far for product demonstrations, training and quotes.
As a result and following the opening of its new 11,000-square-foot manufacturing facility in Tempe, Arizona, in October 2019, Wrap Technologies announced a 352% year-on-year increase in revenues for 3Q2019 – a testament to the growing popularity of its mobile restraint device.
The company expects its growth to continue as adoption rates of the BolaWrap product increase throughout the United States and globally. According to a study by Stratistics MRC, the addressable global market for non-lethal weapons accounted for $6.32 billion in 2016 and is set to rise to $11.85 billion by 2023.
Product Received to Positive Acclaim
- “An innovation that is changing the world of policing.” – Chief Luther Reynolds, Charleston Police Department
- “Anytime you can have a more humane response to someone in crisis, it’s not only good for the department, it’s good for society.” – Redditt Hudson, Regional Field Director of the NAACP (http://nnw.fm/1STXm)
- “This is going to save lives.” – Chief Ed Hudak, Coral Gables Police Department
- “I see this as one of the great tools if you encounter someone with a mental health crisis.” – Chief Steven Casstevens, Buffalo Grove Police Department
Recently completed $12.4 million financing round
Wrap Technologies announced that it had successfully completed its capital raising round on June 4, 2020, raising $12.4 million through a primary share placement priced at $6.00/share. The net proceeds will be use to further scale engineering, fund product development and provide working capital to meet worldwide demand for BolaWrap products and accessories (http://nnw.fm/byLV7). The company also announced that its founder, Elwood Norris, had chosen to exercise 100,000 outstanding warrants to contribute $500,000 to the capital raising efforts. Following the financing round, Wrap Technologies reported over $30 million in cash on hand.
Management Team
Elwood G. “Woody” Norris, Founder and Chief Technology Officer
Elwood G. “Woody” Norris is an award-winning American inventor and serial entrepreneur and currently serves as chief technology officer for Wrap Technologies Inc. Norris founded and served as a director and president of Parametric Sound Corporation (now Turtle Beach Corporation (NASDAQ:HEAR)) and also served as chief scientist at Turtle Beach. Norris previously founded LRAD Corporation (NASDAQ: LRAD) and, prior to retiring in 2010, was chairman of LRAD Corporation’s board of directors, serving as a technical advisor and product spokesperson. Norris has authored more than 80 U.S. patents, primarily in the fields of electrical and acoustical engineering, and has been a frequent speaker on innovation to corporations and government organizations. He is the inventor of Wrap Technologies’ patented and patent pending BolaWrap® technology.
Scot Cohen, Executive Chairman
Scot Cohen has more than 20 years of experience in institutional asset management, wealth management, and capital markets. Cohen founded and served as principal of the Iroquois Capital Opportunity Fund, a closed-end private equity fund which focused on investments in North American oil and gas. Cohen also co-founded Iroquois Capital, a New York-based hedge fund that managed approximately $300 million across its family of funds. Prior to Iroquois Capital, Cohen founded a merchant bank which actively participated in structured investments in public companies. Cohen is currently active on a number of public and private company boards and is involved with various charitable ventures.
David Norris, Chief Executive Officer
David Norris is an experienced executive who joined Wrap Technologies full-time in January 2018. From April 2014 to December 2017, he served in various executive roles, including president, at privately held loanDepot LLC as it rapidly expanded into the fifth largest mortgage lender in the U.S. loanDepot had 6,000 employees and generated $1 billion in revenue in 2017. Norris also served as CEO of Greenlight Financial, and president of LendingTree Loans. Norris’ career also includes executive and management roles at Toshiba America Information Systems and Qualcomm Personal. Earlier in his career, Norris served as a probation officer in San Diego for five years.
Tom Smith, President
Tom Smith co-founded TASER International (now Axon Enterprise Inc. (NASDAQ: AAXN)) (“TASER”) in 1993 and served as president of TASER until October 2006. He served as chairman of the board of directors of TASER from October 2006 until he retired to pursue entrepreneurial activities in February 2012. Amongst his most significant roles and responsibilities at TASER, Smith managed domestic and international sales, significantly expanding the sale and distribution of TASER’s products, including sales to more than 17,200 federal, state and local law enforcement agencies in over 100 countries. In 2012, he founded Achilles Technology Solutions LLC, which, through subsidiary ATS Armor, developed a line of ballistic solutions for law enforcement and military applications. Smith holds a B.S. in ecology and evolutionary biology from the University of Arizona and an M.B.A. from Northern Arizona University.
Jim Barnes, Chief Financial Officer
Jim Barnes has served as president of Sunrise Capital Inc., a private venture capital and financial and regulatory consulting firm, since 1984. Barnes was chief financial officer of Parametric Sound Corporation (now Turtle Beach Corporation), and also served as vice president administration at Turtle Beach Corporation. Since 1999, Barnes has been manager of Syzygy Licensing LLC, a private technology invention and licensing company he owns with Elwood Norris. Barnes previously practiced as a certified public accountant and management consultant with Ernst & Ernst and Touche Ross & Co., and as a principal in J. McDonald & Co. Ltd. in Phoenix, Arizona.
XPhyto Therapeutics Corp. (CSE: XPHY) (OTCQB: XPHYF) (FSE: 4XT)
XPhyto Therapeutics Corp. (CSE: XPHY) (OTCQB: XPHYF) (FSE: 4XT) is a bioscience accelerator focused on next-generation drug delivery, diagnostic and new active pharmaceutical ingredient investment opportunities. This includes products that are being readied for commercialization within the coming weeks, such as a rapid COVID-19 PCR test kit that reduces turnaround times to less than 30 minutes.
The company has research and development operations in North America and Europe and an operational focus in Germany. Its regulatory approval and commercialization focus is currently on products for the European market.
XPhyto was founded in 2017 and is headquartered in Vancouver, British Columbia.
Business Strategy & Milestones for 2021
On January 18, 2021, XPhyto issued a news release detailing its business strategy for the coming year. The company noted that it is “on the cusp of transformational change as product development programs advance from the laboratory to the clinic.” In addition to continuing to leverage its scientific expertise and operations in North America and Europe for product development and optimization, XPhyto intends to pursue growth through the commercialization of existing products and adherence to a focused investment strategy targeting impact-driven innovation with “the potential for extreme value creation.”
In particular, XPhyto is well positioned to execute on opportunities across its current business divisions, including:
- Commercialization of infectious disease diagnostics
- Clinical validation of transdermal and sublingual drug formulations
- Continued investment and development in psychedelic medicine
“2020 was a very productive year for XPhyto. We made significant progress in all areas of our business,” Hugh Rogers, CEO & Director of XPhyto, stated in the update. “We have ambitious milestones for 2021 with multiple product launches on the horizon, multiple clinical drug programs underway, and an aggressive commitment to psychedelic medicine. I am extremely confident that our team can execute on the company’s business plan for 2021.”
Infectious Disease Diagnostics
XPhyto’s lead diagnostic product, secured through an exclusive global commercialization agreement with 3a-diagnostics GmbH (“3a”), is a rapid and highly portable PCR diagnostic test. Notably, PCR testing “has emerged as the only internationally recognized standard for COVID-19 testing” and is expected to play a key role in facilitating the recovery of the domestic and international travel industries, among others.
Successful validation of the PCR system was achieved in Q4 2020, and XPhyto has expressed confidence that it will achieve European commercial (CE-IVD) approval in Q1 2021. In preparation for this milestone and an anticipated Q1 product launch, the company is currently in discussion with manufacturing and distribution partners in Europe and the Middle East.
In addition to COVID-19 products, XPhyto and partner 3a are developing and commercializing a portfolio of low-cost oral biosensors. The company’s lead biosensor product is an oral health screening test for the detection of peri-implantitis for which XPhyto is targeting a late 2021 European commercial approval.
XPhyto does not make any express or implied claims that its product has the ability to eliminate, cure or contain the COVID-19 pandemic.
Drug Formulation & Delivery
In 2020, XPhyto’s German subsidiary, Vektor Pharma TF GmbH (“Vektor”), reported significant advancement in four therapeutic programs targeting neurological indications with significant market demand. Vektor also successfully developed a sublingual drug formulation on contract for a major generic drug manufacturer and distributor.
XPhyto will look to build on this progress in 2021, with plans to complete human pilot studies evaluating its four lead therapeutic products:
- Rotigotine transdermal patch for Parkinson’s disease
- CBD oral/sublingual strip for treatment resistant epilepsy
- THC oral/sublingual strip for anorexia/nausea
- CBD:THC (1:1) oral/sublingual strip for multiple sclerosis associated spasticity
Per its 2021 business update, the company is currently in “ongoing discussions with multiple potential commercial partners, licensors and distributors and will be reviewing monetization opportunities on a continued basis.”
Psychedelic Medicine
Psychedelic compounds are a highly promising new class of active pharmaceutical ingredient (“API”) demonstrating strong potential for a variety of mental health conditions. XPhyto is positioned to capitalize on this promise through two strategic initiatives:
- An agreement for the development of industrial scale biotechnology processes for the production of psilocybin
- An agreement for R&D related to multiple psychedelic compounds, including psilocybin, mescaline, LSD, MDMA and DMT, among others
XPhyto intends to advance and expand its programs focused on the industrial scale production of psychedelic API in 2021. The company also plans to launch new programs for the development of psychedelic drug formulations, with a focus on sublingual and transdermal therapeutics and the integration of these products into established clinical programs relating to mental health indications.
Management Team
Hugh Rogers is the CEO and Director of XPhyto Therapeutics Corp. He is an entrepreneur and lawyer with private and public start-up company experience in various industries and operational roles. His recent advisory work has focused on public listings and corporate restructuring. This restructuring has occurred in the life science (cell therapy and medical device) and natural resources (natural gas co-gen and conventional oil) industries. Mr. Rogers holds a bachelor’s degree in Cellular Biology and Genetics and a law degree. He is a member in good standing of the Law Society of British Colombia.
Christopher Ross is the CFO of XPhyto. He is a professional accountant with broad financial experience across numerous industries, including forestry, distribution, construction, mining and multi-family real estate. He has provided advisory services to private and public companies in the areas of financial accounting, strategic analysis, audit and taxation. Mr. Ross holds a bachelor’s degree in commerce. He is a member in good standing with the Chartered Professional Accountants Association of British Columbia.
Wolfgang Probst serves as Director of XPhyto and Managing Director of BUNKER Pflanzenextrakte GmbH. He is a seasoned management and financial consultant based in Bavaria, Germany. He has consulting experience as branch head working with private clients and corporations of high net worth. In 2017, Mr. Probst assumed the CFO role of BUNKER and continues to play a key role in its operational and financial development.
Professor Dr. Raimar Löbenberg serves as Director of XPhyto. He holds a Bachelor of Science in pharmacy from Johannes Gutenberg-University and a Ph.D. in pharmaceutics from the Johann Wolfgang Goethe-University. He is the co-founder of RS Therapeutics Inc., which concentrates on foam-based topical drug delivery systems.
Professor Dr. Thomas Beckert is the Founder and Managing Director of Vektor Pharma TF GmbH. His expertise includes the formulation and machine development of transdermal therapeutic systems and ODFs. Professor Beckert holds a Bachelor of Science in pharmacy from the University of Freiburg and a Ph.D. in pharmacy and economics from the University of Tubingen.
